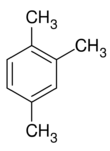
Chemistry:1,2,4-Trimethylbenzene
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2,4-Trimethylbenzene | |||
| Other names
Pseudocumene,
Asymmetrical trimethylbenzene, ψ-Cumene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1903005 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1993 2325 | ||
| |||
| |||
| Properties | |||
| C9H12 | |||
| Molar mass | 120.19 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 0.8761 g/cm3 | ||
| Melting point | −43.78 °C (−46.80 °F; 229.37 K) | ||
| Boiling point | 169 to 171 °C (336 to 340 °F; 442 to 444 K) | ||
| -101.6·10−6 cm3/mol | |||
| Hazards | |||
| Safety data sheet | Sigma-Aldrich MSDS | ||
| GHS pictograms |   
| ||
| GHS Signal word | Warning | ||
| H226, H315, H319, H332, H335, H411 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P273, P280, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P391, P403+233, P403+235 | |||
| Flash point | 44.4 °C (111.9 °F; 317.5 K) | ||
| Explosive limits | 0.9%–6.4%[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[2] | ||
| Related compounds | |||
Related compounds
|
1,2,3-Trimethylbenzene; 1,3,5-Trimethylbenzene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
1,2,4-Trimethylbenzene, also known as pseudocumene, is an organic compound with the chemical formula C6H3(CH3)3. Classified as an aromatic hydrocarbon, it is a flammable colorless liquid with a strong odor. It is nearly insoluble in water but soluble in organic solvents. It occurs naturally in coal tar and petroleum (about 3%). It is one of the three isomers of trimethylbenzene.
Production
Industrially, it is isolated from the C9 aromatic hydrocarbon fraction during petroleum distillation. Approximately 40% of this fraction is 1,2,4-trimethylbenzene. It is also generated by methylation of toluene and xylenes and the disproportionation of xylene over aluminosilicate catalysts.[3]
Uses
Pseudocumene is a precursor to mellitic anhydride, from which high performance polymers are made. It is also used as a sterilizing agent and in the making of dyes, perfumes and resins. Another use is as a gasoline additive.[4]
Scintillator
1,2,4-Trimethylbenzene dissolved in mineral oil is used as a liquid scintillator[5] in particle physics experiments such as NOνA and Borexino.
See also
References
- ↑ Merck Index, 11th Edition, 7929
- ↑ 2.0 2.1 NIOSH Pocket Guide to Chemical Hazards. "#0638". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0638.html.
- ↑ Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke "Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_227
- ↑ "Chemical Summary for 1,2,4-Trimethylbenzene" (text). United States Environmental Protection Agency. 1994-08-01. http://www.epa.gov/chemfact/s_trimet.txt. Retrieved 2008-01-28.
- ↑ Mufson, S. (November 1, 2015). "Liquid scintillator production for the NOvA experiment". Nuclear Instruments and Methods A 799: 1–9. doi:10.1016/j.nima.2015.07.026. Bibcode: 2015NIMPA.799....1M.
External links
 |