Chemistry:Product selectivity
Product selectivity is the ability to favor a certain product over others in an organic reaction where multiple products could potentially be generated from one substrate. Product selectivity includes stereoselectivity and regioselectivity; the former refers to the selectivity for a stereoisomer and the later for constitutional isomer. Product selectivity is usually observed when one reaction pathway is preferred and is often expressed as the ratio of product isomers or the ratio of rate constants for reaction pathways that generate those product isomers.
Control of product selectivity is very important for development and application of organic reactions. For example, the endeavors toward preferentially generating one stereoisomer over others have led to the development of a branch of organic synthesis called asymmetric synthesis or enantioselective synthesis.
A simple example of a regioselective reaction is addition of HBr to 1-hexene. The reaction follows Markovnikov’s rule; the major product has the bromine atom linked to the more substituted carbon.
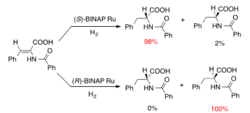
A prominent example to illustrate stereoselectivity in organic chemistry is Noyori asymmetric hydrogenation[1] as shown in Figure 2. The selectivity of hydrogenation is achieved by using different ruthenium-based asymmetric catalysts.
References
- ↑ Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. J. Am. Chem. Soc. 1980, 102, 7932-7934.