Chemistry:N1-Methylpseudouridine
| |
| Names | |
|---|---|
| IUPAC name
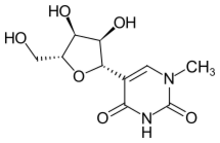
5-[(2S,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1-methylpyrimidine-2,4-dione
| |
| Other names
1-Methylpseudouridine; m1Ψ
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C 10H 14N 2O 6 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N1-Methylpseudouridine (abbreviated m1Ψ) is a natural archaeal tRNA component,[1] component of mammalian ribosomal RNA,[2] and "hypermodified" pyrimidine nucleoside used in biochemistry and molecular biology for in vitro transcription and is found in the SARS-CoV-2 mRNA vaccines tozinameran (Pfizer–BioNTech) and elasomeran (Moderna).[3]
Properties
N1-Methylpseudouridine is the methylated derivative of pseudouridine. It is used in in vitro transcription and for the production of RNA vaccines.[4][5] In vertebrates, it stimulates significantly less activation of the innate immune response compared to uridine,[6] while the translation is stronger.[7][8] In protein biosynthesis, it is read like uridine and enables comparatively high protein yields.[8][9] The nucleoside itself can be made by chemical methylation of pseudouridine.[10]
While pseudouridine can wobble-pair with bases other than A,[11] work examining COVID-19 modRNA vaccines that replace all their uridines with N1-methylpseudouridine show faithful protein production.[12]
More recent work from Mulroney and colleagues has identified that N1-methylpseudouridine can give rise to slippery sequences that promote ribosomal frameshifting.[13] This issue is readily correctable through the replacement of slippery sequences with synonymous codons. The frameshifting not known to contribute to any safety issues with regard to current mRNA vaccines, nor has it been shown to limit their effectiveness. In work from Mulroney and colleagues, mice immunized with the Bnt162b2 vaccine demonstrate a greater T cell response against in-frame spike protein than those receiving Vaxzevria, despite the latter not demonstrating meaningful production of frameshifted sequences. In human donors, the degree of recognition of frameshifted peptides by T cells varies greatly, suggesting that the extent to which frameshifting occurs may vary greatly as well. Importantly, frameshifted products are rare but well-defined events in protein production, including in viral infections, and can give rise to sequences that can be targeted by the immune system.[14][15] Furthermore, despite significant disparity at the level of nucleotide sequences between COVID-19 vaccines from Pfizer/BioNTech and Moderna,[16] the safety profile of both vaccines is comparable,[17] arguing against any meaningful effect of frameshifting on the safety profile of the vaccines.
History
In 2016, a protocol for large-scale synthesis of the nucleoside triphosphate from the ribonucleoside was published.[18]
In 2017–2018 it was tested in vaccines against Zika,[19][20][21] HIV-1,[21] influenza,[21] and Ebola.[22][3]:5
References
- ↑ "Identification of the enzyme responsible for N1-methylation of pseudouridine 54 in archaeal tRNAs". RNA 18 (3): 412–420. March 2012. doi:10.1261/rna.028498.111. PMID 22274954. "In contrast, in most archaea this position is occupied by another hypermodified nucleotide: the isosteric N1-methylated pseudouridine".
- ↑ "Error: no
|title=specified when using {{Cite web}}". https://academic.oup.com/nar/article/38/7/2387/3100516. - ↑ 3.0 3.1 "The Critical Contribution of Pseudouridine to mRNA COVID-19 Vaccines". Frontiers in Cell and Developmental Biology 9: 789427. 2021-11-04. doi:10.3389/fcell.2021.789427. PMID 34805188.
- ↑ "Lipid-nanoparticle-encapsulated mRNA vaccines induce protective memory CD8 T cells against a lethal viral infection". Molecular Therapy 29 (9): 2769–2781. September 2021. doi:10.1016/j.ymthe.2021.05.011. PMID 33992803.
- ↑ "A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis". Science 371 (6525): 145–153. January 2021. doi:10.1126/science.aay3638. PMID 33414215. Bibcode: 2021Sci...371..145K.
- ↑ "Impact of mRNA chemistry and manufacturing process on innate immune activation". Science Advances 6 (26): eaaz6893. June 2020. doi:10.1126/sciadv.aaz6893. PMID 32637598. Bibcode: 2020SciA....6.6893N.
- ↑ "N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice". Journal of Controlled Release 217: 337–344. November 2015. doi:10.1016/j.jconrel.2015.08.051. PMID 26342664. https://biblio.ugent.be/publication/6993270.
- ↑ 8.0 8.1 "N1-methyl-pseudouridine in mRNA enhances translation through eIF2α-dependent and independent mechanisms by increasing ribosome density". Nucleic Acids Research 45 (10): 6023–6036. June 2017. doi:10.1093/nar/gkx135. PMID 28334758.
- ↑ "N 1-Methylpseudouridine substitution enhances the performance of synthetic mRNA switches in cells". Nucleic Acids Research 48 (6): e35. April 2020. doi:10.1093/nar/gkaa070. PMID 32090264.
- ↑ "A chemical synthesis of the nucleoside 1-methylpseudouridine". Journal of Heterocyclic Chemistry 14 (4): 699–700. June 1977. doi:10.1002/jhet.5570140437.
- ↑ "The contribution of pseudouridine to stabilities and structure of RNAs". Nucleic Acids Research 42 (5): 3492–3501. March 2014. doi:10.1093/nar/gkt1330. PMID 24369424.
- ↑ Kim, Kyusik Q.; Burgute, Bhagyashri D.; Tzeng, Shin-Cheng; Jing, Crystal; Jungers, Courtney; Zhang, Junya; Yan, Liewei L.; Vierstra, Richard D. et al. (2022-08-30). "N1-methylpseudouridine found within COVID-19 mRNA vaccines produces faithful protein products". Cell Reports 40 (9): 111300. doi:10.1016/j.celrep.2022.111300. ISSN 2211-1247. PMID 35988540.
- ↑ Mulroney, Thomas E.; Pöyry, Tuija; Yam-Puc, Juan Carlos; Rust, Maria; Harvey, Robert F.; Kalmar, Lajos; Horner, Emily; Booth, Lucy et al. (2023-12-06). "N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting" (in en). Nature 625 (7993): 189–194. doi:10.1038/s41586-023-06800-3. ISSN 1476-4687. PMID 38057663.
- ↑ Hogan, Michael J.; Maheshwari, Nikita; Begg, Bridget E.; Nicastri, Annalisa; Hedgepeth, Emma J.; Muramatsu, Hiromi; Pardi, Norbert; Miller, Michael A. et al. (November 2023). "Cryptic MHC-E epitope from influenza elicits a potent cytolytic T cell response" (in en). Nature Immunology 24 (11): 1933–1946. doi:10.1038/s41590-023-01644-5. ISSN 1529-2916. PMID 37828378. https://www.nature.com/articles/s41590-023-01644-5.
- ↑ Dolan, Brian P.; Li, Lily; Takeda, Kazuyo; Bennink, Jack R.; Yewdell, Jonathan W. (2010-02-01). "Defective Ribosomal Products Are the Major Source of Antigenic Peptides Endogenously Generated from Influenza A Virus Neuraminidase". Journal of Immunology 184 (3): 1419–1424. doi:10.4049/jimmunol.0901907. ISSN 0022-1767. PMID 20038640.
- ↑ Zhang, Lizhou; More, Kunal R.; Ojha, Amrita; Jackson, Cody B.; Quinlan, Brian D.; Li, Hao; He, Wenhui; Farzan, Michael et al. (2023-10-11). "Effect of mRNA-LNP components of two globally-marketed COVID-19 vaccines on efficacy and stability" (in en). npj Vaccines 8 (1): 156. doi:10.1038/s41541-023-00751-6. ISSN 2059-0105. PMID 37821446.
- ↑ Dickerman, Barbra A.; Madenci, Arin L.; Gerlovin, Hanna; Kurgansky, Katherine E.; Wise, Jessica K.; Figueroa Muñiz, Michael J.; Ferolito, Brian R.; Gagnon, David R. et al. (2022-07-01). "Comparative Safety of BNT162b2 and mRNA-1273 Vaccines in a Nationwide Cohort of US Veterans". JAMA Internal Medicine 182 (7): 739–746. doi:10.1001/jamainternmed.2022.2109. ISSN 2168-6106. PMID 35696161. PMC 9194743. https://doi.org/10.1001/jamainternmed.2022.2109.
- ↑ "Gram-Scale Chemical Synthesis of Base-Modified Ribonucleoside-5'-O-Triphosphates". Current Protocols in Nucleic Acid Chemistry 67: 13.15.1–13.15.10. December 2016. doi:10.1002/cpnc.20. PMID 27911496.
- ↑ "Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination". Nature 543 (7644): 248–251. March 2017. doi:10.1038/nature21428. PMID 28151488. Bibcode: 2017Natur.543..248P. "we designed a potent anti-ZIKV vaccine … containing the modified nucleoside 1-methylpseudouridine (m1Ψ)".
- ↑ "Modified mRNA Vaccines Protect against Zika Virus Infection". Cell 168 (6): 1114–1125.e10. March 2017. doi:10.1016/j.cell.2017.02.017. PMID 28222903. "The mRNA was synthesized … where the UTP was substituted with 1-methylpseudoUTP".
- ↑ 21.0 21.1 21.2 "Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses". The Journal of Experimental Medicine 215 (6): 1571–1588. June 2018. doi:10.1084/jem.20171450. PMID 29739835. "In this study, we characterize the immunogenicity of three vaccines consisting of m1Ψ-modified, FPLC-purified mRNA-LNPs encoding HIV-1 envelope (Env), ZIKV prM-E, and influenza virus hemagglutinin (HA)".
- ↑ "Modified mRNA-Based Vaccines Elicit Robust Immune Responses and Protect Guinea Pigs From Ebola Virus Disease". The Journal of Infectious Diseases 217 (3): 451–455. January 2018. doi:10.1093/infdis/jix592. PMID 29281112. "Two mRNA vaccines were synthesized … where the UTP were substituted with 1-methylpseudo UTP".
 |

