Chemistry:Kappa-KTx2.5
κ-KTx2.5 is a toxin found in the venom of the scorpion, Opisthacanthus cayaporum. The toxin belongs to the κ-KTx family, a channel blocker family that targets voltage-gated potassium channels (Kv) 1.1 and 1.4.
Etymology
The toxin κ-KTx2.5 is part of the κ-family of potassium-channel toxins (κ-KTxs),[1] and was identified in the scorpion Opisthacanthus cayaporum. The first κ-KTx were isolated from the Scorpionidae Heterometrus fulvipes[2] and systematically named κ-KTx1.n, being 'n' the order number in which they were found and described. Later, a second group of potassium channel toxins were isolated from another scorpium genus, Opisthacanthus, and called Om-toxins. The first ones found were from Opisthacanthus madagascariensis[3] and named κ-KTx2.1 to κ-KTx2.4 .
κ-KTx2.5 is the mature peptide coded by the sequence OcyC8,[4] which was isolated from Opisthacanthus cayaporum[5] and named after the species (Ocy) and clone number (C8).
Source
κ-KTx2.5 is a toxin that is derived from the venom of Opisthacanthus cayaporum[1], also known as the South American scorpion.[6] Scorpion is the main source of potassium-channel toxins among spiders,[7][8] snakes,[9] cone-snails,[10][11] and sea anemones[12][13]
Chemistry
Structure
The κ-KTx2.5 toxin is a 28 amino acid long peptide with two disulfide bonds.[1] The functional dyad (pair of amino acid residues that are involved in recognition and blocking of specific channels[14]) of KTxs is composed of two amino acid residues, a lysine residue and an aromatic residue (tyrosine or phenylalanine).[14]
By comparison, the κ-KTx2.5 possesses a valine residue in the position corresponding to tyrosine residue (the aromatic residue), and an arginine instead of the lysine residue.[15] The peptide is similarly stabilized by two disulfide bonds.
The amino acid sequence of κ-KTx2.5 is: YDACVNACLEHHPNVRECEEACKNPVPP[1]
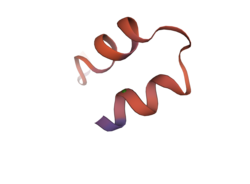
Homology
In Basic Local Alignment Search Tool (BLAST), κ-KTx2.5 is shown to exhibit sequence homology with 4 other toxins and the calcium release-activated calcium channel protein 1 (with 28% identification).[4][17] κ-KTx2.5 shows highest sequence identity (66.67%) with κ-KTx2.9, extracted from another species of scorpion, Pandinus imperator.[4][17] Other toxins with sequence homology to κ-KTx2.5 are (Om-toxins) OmTx2, OmTx1, and OmTx3 with sequence identities of 64%, 64%, and 45.45%, respectively.
Family
The κ-KTx2.5 belongs to a potassium-channel blocker family KTx.[1] Most peptides in KTxs family share common residues that facilitate binding with the potassium-channel vestibule.[1] These peptides are formed by 20 to 95 amino acid residues and are stabilized by disulfide bonds.[1]
κ-KTxs were classified as a separate family from the α, β, and γ families of KTxs, which were identified by their highly conserved secondary structures composed of an α helix and a β sheet (α/β).[18] κ-KTxs differ in their structural arrangement from α, β, and γ-KTxs as their secondary structures are composed of two α-helices (α/α).[2][3]
Target
κ-KTx2.5 is capable of reversibly blocking voltage gated potassium channels (Kv), while it was shown to have no effect on voltage gated sodium channels (Nav).[1] The toxins' main targets are Kv1.1 (IC50 = 46 µM) and Kv1.4 (IC50 = 71 µM), showing a low affinity for Kv channels overall. κ-KTx2.5 (at 16 µM) reduced potassium currents through Kv1.4 (by 50%) and Kv1.1 (by 20%).[1]
Mode of Action
κ-KTx2.5 binds potassium-channels through the interaction between the toxin’s C-terminal and the extracellular loop between transmembrane segments S5 and S6 of the potassium channel.[1] On the level of amino acids, the asparagine-24 (N24) residue of κ-KTx2.5 interacts with aspartic acid-348 (D348) of Kv1.2 at a distance of 3.7 Å, affecting only one subunit.[1]
Lysine-23 (K23) in the toxin likely aids in anchoring to the potassium-channel, with an interaction distance of 5.1 Å between the toxin K23 and channel D348.[1] The introduction of a second κ-KTx2.5 toxin leads to interactions with other channel subunits,[1] potentially blocking the channel pore through toxin-toxin interactions.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Camargos, Thalita Soares; Restano-Cassulini, Rita; Possani, Lourival Domingos; Peigneur, Steve; Tytgat, Jan; Schwartz, Carlos Alberto; Alves, Erica Maria C; de Freitas, Sonia Maria et al. (July 2011). "The new kappa-KTx 2.5 from the scorpion Opisthacanthus cayaporum" (in en). Peptides 32 (7): 1509–1517. doi:10.1016/j.peptides.2011.05.017. PMID 21624408. https://linkinghub.elsevier.com/retrieve/pii/S0196978111002099.
- ↑ 2.0 2.1 Srinivasan, Kellathur N.; Sivaraja, Vaithiyalingam; Huys, Isabelle; Sasaki, Toru; Cheng, Betty; Kumar, Thallampuranam Krishnaswamy S.; Sato, Kazuki; Tytgat, Jan et al. (August 2002). "κ-Hefutoxin1, a Novel Toxin from the ScorpionHeterometrus fulvipes with Unique Structure and Function" (in en). Journal of Biological Chemistry 277 (33): 30040–30047. doi:10.1074/jbc.M111258200. PMID 12034709.
- ↑ 3.0 3.1 Chagot, Benjamin; Pimentel, Cyril; Dai, Li; Pil, Joost; Tytgat, Jan; Nakajima, Terumi; Corzo, Gerardo; Darbon, Hervé et al. (2005-05-15). "An unusual fold for potassium channel blockers: NMR structure of three toxins from the scorpion Opisthacanthus madagascariensis" (in en). Biochemical Journal 388 (1): 263–271. doi:10.1042/BJ20041705. ISSN 0264-6021. PMID 15631621. PMC 1186715. https://portlandpress.com/biochemj/article/388/1/263/41928/An-unusual-fold-for-potassium-channel-blockers-NMR.
- ↑ 4.0 4.1 4.2 Altschul, S. (1997-09-01). "Gapped BLAST and PSI-BLAST: a new generation of protein database search programs". Nucleic Acids Research 25 (17): 3389–3402. doi:10.1093/nar/25.17.3389. PMID 9254694.
- ↑ Silva, Édelyn C.N.; Camargos, Thalita S.; Maranhão, Andrea Q.; Silva-Pereira, Ildinete; Silva, Luciano P.; Possani, Lourival D.; Schwartz, Elisabeth F. (September 2009). "Cloning and characterization of cDNA sequences encoding for new venom peptides of the Brazilian scorpion Opisthacanthus cayaporum". Toxicon 54 (3): 252–261. doi:10.1016/j.toxicon.2009.04.010. ISSN 0041-0101. PMID 19379768. http://dx.doi.org/10.1016/j.toxicon.2009.04.010.
- ↑ "UniProt". https://www.uniprot.org/taxonomy/573324.
- ↑ Estrada, Georgina; Villegas, Elba; Corzo, Gerardo (2007). "Spider venoms: a rich source of acylpolyamines and peptides as new leads for CNS drugs" (in en). Nat. Prod. Rep. 24 (1): 145–161. doi:10.1039/B603083C. ISSN 0265-0568. PMID 17268611. http://xlink.rsc.org/?DOI=B603083C.
- ↑ Swartz, Kenton J. (Feb 2007). "Tarantula toxins interacting with voltage sensors in potassium channels". Toxicon 49 (2): 213–230. doi:10.1016/j.toxicon.2006.09.024. ISSN 0041-0101. PMID 17097703. PMC 1839852. http://dx.doi.org/10.1016/j.toxicon.2006.09.024.
- ↑ Harvey, Alan L. (January 1997). "Recent studies on dendrotoxins and potassium ion channels". General Pharmacology: The Vascular System 28 (1): 7–12. doi:10.1016/s0306-3623(96)00173-5. ISSN 0306-3623. PMID 9112070. http://dx.doi.org/10.1016/s0306-3623(96)00173-5.
- ↑ Han, Tiffany; Teichert, Russell; Olivera, Baldomero; Bulaj, Grzegorz (2008-08-01). "Conus Venoms - A Rich Source of Peptide-Based Therapeutics". Current Pharmaceutical Design 14 (24): 2462–2479. doi:10.2174/138161208785777469. ISSN 1381-6128. PMID 18781995. http://dx.doi.org/10.2174/138161208785777469.
- ↑ """". Expert Opinion on Drug Discovery 2 (9). September 2007. doi:10.1517/edc.2007.2.issue-9. ISSN 1746-0441. http://dx.doi.org/10.1517/edc.2007.2.issue-9.
- ↑ Castañeda, Olga; Harvey, Alan L. (December 2009). "Discovery and characterization of cnidarian peptide toxins that affect neuronal potassium ion channels". Toxicon 54 (8): 1119–1124. doi:10.1016/j.toxicon.2009.02.032. ISSN 0041-0101. PMID 19269305. http://dx.doi.org/10.1016/j.toxicon.2009.02.032.
- ↑ "Expansion ofPMBR.ElecEd". Plant Molecular Biology Reporter 12 (2): 99. June 1994. doi:10.1007/bf02668367. ISSN 0735-9640. http://dx.doi.org/10.1007/bf02668367.
- ↑ 14.0 14.1 Mouhat, Stephanie; De Waard, Michel; Sabatier, Jean‐Marc (February 2005). "Contribution of the functional dyad of animal toxins acting on voltage‐gated Kv1‐type channels" (in en). Journal of Peptide Science 11 (2): 65–68. doi:10.1002/psc.630. ISSN 1075-2617. PMID 15635666. https://onlinelibrary.wiley.com/doi/10.1002/psc.630.
- ↑ Dauplais, Marc; Lecoq, Alain; Song, Jianxing; Cotton, Joël; Jamin, Nadège; Gilquin, Bernard; Roumestand, Christian; Vita, Claudio et al. (February 1997). "On the Convergent Evolution of Animal Toxins". Journal of Biological Chemistry 272 (7): 4302–4309. doi:10.1074/jbc.272.7.4302. ISSN 0021-9258. PMID 9020148.
- ↑ "P86110 | SWISS-MODEL Repository". https://swissmodel.expasy.org/repository/uniprot/P86110.
- ↑ 17.0 17.1 Altschul, Stephen F.; Wootton, John C.; Gertz, E. Michael; Agarwala, Richa; Morgulis, Aleksandr; Schaffer, Alejandro A.; Yu, Yi-Kuo (October 2005). "Protein database searches using compositionally adjusted substitution matrices" (in en). FEBS Journal 272 (20): 5101–5109. doi:10.1111/j.1742-4658.2005.04945.x. ISSN 1742-464X. PMID 16218944.
- ↑ Tytgat, Jan; Chandy, K.George; Garcia, Maria L; Gutman, George A; Martin-Eauclaire, Marie-France; van der Walt, Jurg J; Possani, Lourival D (November 1999). "A unified nomenclature for short-chain peptides isolated from scorpion venoms: α-KTx molecular subfamilies". Trends in Pharmacological Sciences 20 (11): 444–447. doi:10.1016/s0165-6147(99)01398-x. ISSN 0165-6147. PMID 10542442. http://dx.doi.org/10.1016/s0165-6147(99)01398-x.
 |

