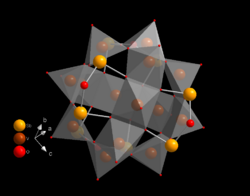
Chemistry:Heteropolymetalate
In chemistry, the heteropolymetalates are a subset of the polyoxometalates, which consist of three or more transition metal oxyanions linked together by shared oxygen atoms to form a closed 3-dimensional molecular framework. In contrast to isopolymetalates, which contain only one kind of metal atom, the heteropolymetalates contain differing main group oxyanions. The metal atoms are usually group 6 (Mo, W) or less commonly group 5 (V, Nb, Ta) transition metals in their highest oxidation states. They are usually colorless to orange, diamagnetic anions. For most heteropolymetalates the W, Mo, or V, is complemented by main group oxyanions phosphate and silicate. Many exceptions to these general statements exist, and the class of compounds includes hundreds of examples. [1][2]
Structure
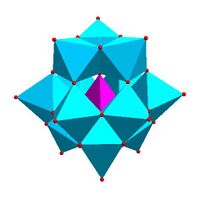
Certain structural motifs recur. The Keggin ion for example is common to both molybdates and tungstates with diverse central heteroatoms. The Keggin and Dawson structures have tetrahedrally-coordinated heteroatoms, such as P or Si, and the Anderson structure[3] has an octahedral central atom, such as aluminium.

|
|

| |
| Strandberg structure, [HP 2Mo 5O 23]4− |
Keggin structure, [XM 12O 40]n − |
Dawson structure, [X 2M 18O 62]n − | |

|

|

|

|
| Anderson structure, [XM 6O 24]n − |
Allman–Waugh structure, [XM 9O 32]n − |
Weakley–Yamase structure, [XM 10O 36]n − |
Dexter–Silverton structure, [XM 12O 42]n − |
Heteropolyacids
Generally, the heteropolymetalates are more thermally robust than homopolymetalates. This trend reflects the stabilizing influence of the tetrahedral oxyanion that "glues" together the transition metal oxo framework. One reflection of their ruggedness, heteropolymetalates can be isolated in their acid form, whereas homopolymetalates typically cannot. Examples include:[4][5]
- Silicotungstic acid, H
4SiW
12O
40 · nH
2O - Phosphomolybdic acid, H
3Mo
12PO
40 · nH
2O - Phosphotungstic acid, H
3W
12PO
40 · nH
2O
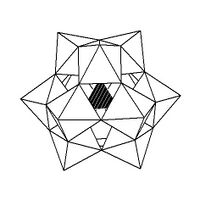
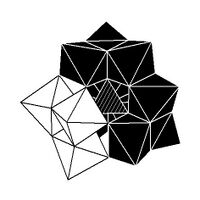
Isomerism
The Keggin structure has 5 isomers, which are obtained by (conceptually) rotating one or more of the four M
3O
13 units through 60°.[citation needed]
| α-[XM 12O 40]n − |
β-[XM 12O 40]n − |
γ-[XM 12O 40]n − |
δ-[XM 12O 40]n − |
ε-[XM 12O 40]n − |
|---|---|---|---|---|
|

|

|
|

|
Lacunary structures
The structure of some POMs are derived from a larger POM's structure by removing one or more addenda atoms and their attendant oxide ions, giving a defect structure called a lacunary structure. An example of a compound with a Dawson lacunary structure is As
2W
15O
56.[6] In 2014, vanadate species with similar, selective metal-binding properties were reported.[7]
Uses
This type of acid is a common re-usable acid catalyst in chemical reactions.[8]
The heteropolyacids are widely used as homogeneous and heterogeneous catalysts,[9] particularly those based on the Keggin structure as they can possess qualities such as good thermal stability, high acidity and high oxidising ability. Some examples of catalysis are:[10]
- Homogeneous acid catalysis
- hydrolysis of propene to give propan-2-ol by H
3PMo
12O
40 and H
3PW
12O
40 - Prins reaction by H
3PW
12O
40 - polymerisation of THF by H
3PW
12O
40
- hydrolysis of propene to give propan-2-ol by H
- Heterogeneous acid catalysis
- Homogeneous oxidation
- cyclohexene + H
2O
2 to adipic acid by the mixed addenda H
3PMo
6V
6O
40 - ketone by O
2 to acid and aldehyde by mixed addenda H
5PMo
10V
2O
40
- cyclohexene + H
Heteropolyacids have long been used in analysis and histology and are a component of many reagents e.g. the Folin-Ciocalteu reagent, folins phenol reagent used in the Lowry protein assay and EPTA, ethanolic phosphotungstic acid.
See also
Citations
- ↑ Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 978-0-7506-3365-9.
- ↑ Pope, M. T. (1983). Heteropoly and Isopoly Oxometalates. New York: Springer Verlag.
- ↑ Blazevic, Amir; Rompel, Annette (January 2016). "The Anderson–Evans polyoxometalate: From inorganic building blocks via hybrid organic–inorganic structures to tomorrows "Bio-POM"" (in en). Coordination Chemistry Reviews 307: 42–64. doi:10.1016/j.ccr.2015.07.001.
- ↑ Dias, J. A.; Dias, S. C. L.; Caliman, E. (2014). "Keggin Structure Polyoxometalates". Keggin Structure Polyoxoometalates. Inorganic Syntheses. 36. p. 210-217. doi:10.1002/9781118744994.ch39. ISBN 9781118744994.
- ↑ Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY.
- ↑ Mbombekalle, I. M.; Keita, B.; Nadjo, L.; Berthet, P.; Neiwert, W. A.; Hill, C. L.; Ritorto, M. D.; Anderson, T. M. (2003). "Manganous heteropolytungstates. Synthesis and heteroatom effects in Wells–Dawson-derived sandwich complexes". Dalton Trans. 2003 (13): 2646–2650. doi:10.1039/b304255c.
- ↑ Kastner, K.; Margraf, J. T.; Clark, T.; Streb, C. (2014). "A Molecular Placeholder Strategy To Access a Family of Transition-Metal-Functionalized Vanadium Oxide Clusters". Chem. Eur. J. 20 (38): 12269–12273. doi:10.1002/chem.201403592. PMID 25082170.
- ↑ Mizuno, Noritaka; Misono, Makoto (1998). "Heterogeneous Catalysis". Chemical Reviews 98: 199–217. doi:10.1021/cr960401q. PMID 11851503.
- ↑ Kozhevnikov, I. V. (1998). "Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions". Chemical Reviews 98 (1): 171–198. doi:10.1021/cr960400y. PMID 11851502.
- ↑ "Oxide catalysts in solid state chemistry". T Okuhara, M Misono. Encyclopedia of Inorganic Chemistry. Editor R Bruce King (1994). John Wiley and Sons. ISBN:0-471-93620-0
References
 |