Chemistry:Europium(II) bromide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Europium(II) bromide
| |
| Other names
Europium dibromide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| EuBr2 | |
| Molar mass | 311.77g[1] |
| Appearance | White Crystalline Solid |
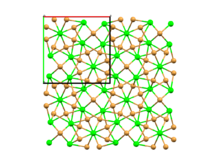
| Structure | |
| SrBr2[2] | |
| Mixed 8 and 7 | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319[1] | |
| P305+351+338[1]P264, P280, P302, P352, P321, P332, P313, P337, P362[3] | |
| Related compounds | |
Other anions
|
Europium(II) chloride Europium(II) fluoride |
Related compounds
|
Europium(III) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Europium(II) bromide is a crystalline compound of one europium atom and two bromine atoms. Europium(II) bromide is a white powder at room temperature,[4] and odorless.[5] Europium dibromide is hygroscopic.[6]
Reactions
Europium(II) bromide is known to be involved in three reactions:[7]
- 2 EuBr3 + Eu → 3 EuBr2 (requires a temperature of 800-900 °C)
- 2 EuBr3 → 2 EuBr2 + Br2 (requires a temperature of 900-1000 °C)
- Eu + HgBr2 → EuBr2 + Hg (requires a temperature of 700-800 °C)
References
- ↑ 1.0 1.1 1.2 "Europium(II) bromide 99.99% trace metals basis | Sigma-Aldrich". http://www.sigmaaldrich.com/catalog/product/aldrich/751936?lang=en®ion=US.
- ↑ Sass, Ronald L.; Brackett, Thomas; Brackett, Elizabeth (December 1963). "THE CRYSTAL STRUCTURE OF STRONTIUM BROMIDE". The Journal of Physical Chemistry 67 (12): 2862–2863. doi:10.1021/j100806a516.
- ↑ "MSDS - 751936". http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=751936&brand=ALDRICH&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2F751936%3Flang%3Den.
- ↑ "Yunnan Titan New Materials Technology Co., Ltd". http://en.allinorganics.com/doc/new_pro/?id=1439.
- ↑ "Europium(II) bromide, White crystalline powder, 99.99% (Metals...". http://www.fishersci.co.uk/shop/products/europium-ii-bromide-white-crystalline-powder-99-99-metals-basis-alfa-aesar-3/p-4781898#tab2.
- ↑ "Europium(II) bromide, 99.99% (metals basis) | VWR". https://us.vwr.com/store/product/7985264/europium-ii-bromide-99-99-metals-basis.
- ↑ "CharChem. Br2Eu". http://easychem.org/en/subst-ref?keyword=Br2Eu&brutto=Br2Eu&langMode=&langs=.
 |

