Chemistry:Dithionitronium hexafluoroarsenate
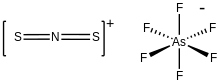
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| AsF6N2S | |
| Molar mass | 248.99 g·mol−1 |
| Appearance | yellow solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dithionitronium hexafloroarsenate is the inorganic compound with the formula [SN
2]AsF
6. It is the hexafluoroarsenate (AsF−
6) salt of S=N=S+. The cation is of interest as the sulfur analogue of nitronium (NO+
2). Hexafloroarsenate is a weakly coordinating anion. According to X-ray crystallography, S=N=S+ is linear with S-N distances of 146 picometers.[1]
Synthesis and reactions
Dithionitronium hexafluoroarsenate is prepared from thiazyl chloride using silver hexafluoroarsenate.[2] The hexachloroantimonate salt can be prepared by treating thiazyl chloride with sulfur in the presence of antimony pentachloride according to this idealized equation:
- SNCl + S + SbCl
5 → [NS
2]SbCl
6
The dithionitronium cation reacts with nitriles to give dithiadiazolium salts:[3]
- [NS
2]+
+ RCN → [RCN
2S
2]+
Addition to alkynes gives dithiazolium salts:
- [NS
2]+
+ R
2C
2 → [(RC)
2NS
2]+
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Ayres, Bryan; Banister, Arthur J.; Coates, Philip D.; Hansford, Michael I.; Rawson, Jeremy M.; Rickard, Clifton E. F.; Hursthouse, Michael B.; Malik, K. M. Abdul et al. (1992). "A General Route to N(SCl)2+ and SNS+ Salts". Journal of the Chemical Society, Dalton Transactions (21): 3097. doi:10.1039/DT9920003097.
- ↑ Rawson, Jeremy M.; Banister, Arthur J.; Lavender, Ian (1995). "The Chemistry of Dithiadiazolylium and Dithiadiazolyl Rings". Adv. Heterocyc. Chem. 62. doi:10.1016/S0065-2725(08)60422-5.
 |

