Chemistry:Cyclopropane
|
| |||
|
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclopropane[2] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C3H6 | |||
| Molar mass | 42.08 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | Sweet | ||
| Density | 1.879 g/L (1 atm, 0 °C) | ||
| Melting point | −128 °C (−198 °F; 145 K) | ||
| Boiling point | −33 °C (−27 °F; 240 K) | ||
| Acidity (pKa) | ~46 | ||
| -39.9·10−6 cm3/mol | |||
| Hazards | |||
| Main hazards | Highly flammable Asphyxiant | ||
| Safety data sheet | External MSDS | ||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives - cyclopropanes - are of commercial or biological significance.[3]
Cyclopropane was used as a clinical inhalational anesthetic from the 1930s through the 1980s. Its high flammability posed a risk of fire and even explosions in the operating room.
History
Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper.[4] Freund treated 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane.[5] The yield of the reaction was improved by Gustavson in 1887 with the use of zinc instead of sodium.[6] Cyclopropane had no commercial application until Henderson and Lucas discovered its anaesthetic properties in 1929;[7] industrial production had begun by 1936.[8] In modern anaesthetic practice, it has been superseded by other agents.
Anaesthesia
Cyclopropane was introduced into clinical use by the American anaesthetist Ralph Waters who used a closed system with carbon dioxide absorption to conserve this then-costly agent. Cyclopropane is a relatively potent, non-irritating and sweet smelling agent with a minimum alveolar concentration of 17.5%[9] and a blood/gas partition coefficient of 0.55. This meant induction of anaesthesia by inhalation of cyclopropane and oxygen was rapid and not unpleasant. However at the conclusion of prolonged anaesthesia patients could suffer a sudden decrease in blood pressure, potentially leading to cardiac dysrhythmia: a reaction known as "cyclopropane shock".[10] For this reason, as well as its high cost and its explosive nature,[11] it was latterly used only for the induction of anaesthesia, and has not been available for clinical use since the mid-1980s. Cylinders and flow meters were colored orange.
Pharmacology
Cyclopropane is inactive at the GABAA and glycine receptors, and instead acts as an NMDA receptor antagonist.[12][13] It also inhibits the AMPA receptor and nicotinic acetylcholine receptors, and activates certain K2P channels.[12][13][14]
Structure and bonding
The triangular structure of cyclopropane requires the bond angles between carbon-carbon covalent bonds to be 60°. The molecule has D3h molecular symmetry. The C-C distances are 151 pm versus 153-155 pm.[15][16]
Despite their shortness, the C-C bonds in cyclopropane are weakened by 34 kcal/mol vs ordinary C-C bonds. In addition to ring strain, the molecule also has torsional strain due to the eclipsed conformation of its hydrogen atoms. The C-H bonds in cyclopropane are stronger than ordinary C-H bonds as reflected by NMR coupling constants.
Bonding between the carbon centres is generally described in terms of bent bonds.[17] In this model the carbon-carbon bonds are bent outwards so that the inter-orbital angle is 104°.
The unusual structural properties of cyclopropane have spawned many theoretical discussions. One theory invokes σ-aromaticity: the stabilization afforded by delocalization of the six electrons of cyclopropane's three C-C σ bonds to explain why the strain of cyclopropane is "only" 27.6 kcal/mol as compared to cyclobutane (26.2 kcal/mol) with cyclohexane as reference with Estr=0 kcal/mol,[18][19][20] in contrast to the usual π aromaticity, that, for example, has a highly stabilizing effect in benzene. Other studies do not support the role of σ-aromaticity in cyclopropane and the existence of an induced ring current; such studies provide an alternative explanation for the energetic stabilization and abnormal magnetic behaviour of cyclopropane.[21]
Synthesis
Cyclopropane was first produced via a Wurtz coupling, in which 1,3-dibromopropane was cyclised using sodium.[4] The yield of this reaction can be improved by the use of zinc as the dehalogenating agent and sodium iodide as a catalyst.[22]
- BrCH2CH2CH2Br + 2 Na → (CH2)3 + 2 NaBr
The preparation of cyclopropane rings is referred to as cyclopropanation.
Reactions
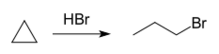
Owing to the increased π-character of its C-C bonds, cyclopropane is often assumed to add bromine to give 1,3-dibromopropane, but this reaction proceeds poorly.[23] Hydrohalogenation with hydrohalic acids gives linear 1-halogenopropanes. Substituted cyclopropanes also react, following Markovnikov's rule.[24]
Cyclopropane and its derivatives can oxidatively add to transition metals, in a process referred to as C–C activation.
Safety
Cyclopropane is highly flammable. However, despite its strain energy it does not exhibit explosive behavior substantially different from other alkanes.
See also
- Tetrahedrane contains four fused cyclopropane rings that form the faces of a tetrahedron
- Propellane contains three cyclopropane rings that share a single central carbon-carbon bond.
- Cyclopropene
- Methylenecyclopropane
References
- ↑ Merck Index, 11th Edition, 2755.
- ↑ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 137. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ Faust, Rüdiger (2001). "Fascinating Natural and Artificial Cyclopropane Architectures". Angewandte Chemie International Edition 40 (12): 2251–2253. doi:10.1002/1521-3773(20010618)40:12<2251::AID-ANIE2251>3.0.CO;2-R. PMID 11433485.
- ↑ 4.0 4.1 August Freund (1881). "Über Trimethylen". Journal für Praktische Chemie 26 (1): 367–377. doi:10.1002/prac.18820260125. https://books.google.com/books?id=WSzzAAAAMAAJ&pg=PA367.
- ↑ August Freund (1882). "Über Trimethylen". Monatshefte für Chemie 3 (1): 625–635. doi:10.1007/BF01516828. https://books.google.com/books?id=b448AAAAIAAJ&pg=PA625.
- ↑ G. Gustavson (1887). "Ueber eine neue Darstellungsmethode des Trimethylens". Journal für Praktische Chemie 36: 300–305. doi:10.1002/prac.18870360127. http://gallica.bnf.fr/ark:/12148/bpt6k90799n/f308.table.
- ↑ G. H. W. Lucas; V. E. Henderson (1 August 1929). "A New Anesthetic: Cyclopropane : A Preliminary Report". Can Med Assoc J 21 (2): 173–5. PMID 20317448.
- ↑ H. B. Hass; E. T. McBee; G. E. Hinds (1936). "Synthesis of Cyclopropane". Industrial & Engineering Chemistry 28 (10): 1178–81. doi:10.1021/ie50322a013.
- ↑ Eger, Edmond I.; Brandstater, Bernard; Saidman, Lawrence J.; Regan, Michael J.; Severinghaus, John W.; Munson, Edwin S. (1965). "Equipotent Alveolar Concentrations of Methoxyflurane, Halothane, Diethyl Ether, Fluroxene, Cyclopropane, Xenon and Nitrous Oxide in the Dog". Anesthesiology 26 (6): 771–777. doi:10.1097/00000542-196511000-00012. PMID 4378907.
- ↑ JOHNSTONE, M; Alberts, JR (July 1950). "Cyclopropane anesthesia and ventricular arrhythmias.". British Heart Journal 12 (3): 239–44. doi:10.1136/hrt.12.3.239. PMID 15426685.
- ↑ MacDonald, AG (June 1994). "A short history of fires and explosions caused by anaesthetic agents.". British Journal of Anaesthesia 72 (6): 710–22. doi:10.1093/bja/72.6.710. PMID 8024925.
- ↑ 12.0 12.1 Hugh C. Hemmings; Philip M. Hopkins (2006). Foundations of Anesthesia: Basic Sciences for Clinical Practice. Elsevier Health Sciences. pp. 292–. ISBN 978-0-323-03707-5. https://books.google.com/books?id=xaXu1wHmENoC&pg=PA292.
- ↑ 13.0 13.1 Hemmings, Hugh C. (2009). "Molecular Targets of General Anesthetics in the Nervous System". Suppressing the Mind: 11–31. doi:10.1007/978-1-60761-462-3_2. ISBN 978-1-60761-463-0.
- ↑ "Nonhalogenated alkanes cyclopropane and butane affect neurotransmitter-gated ion channel and G-protein-coupled receptors: differential actions on GABAA and glycine receptors". Anesthesiology 97 (6): 1512–20. December 2002. doi:10.1097/00000542-200212000-00025. PMID 12459679.[yes|permanent dead link|dead link}}]
- ↑ Allen, Frank H.; Kennard, Olga; Watson, David G.; Brammer, Lee; Orpen, A. Guy; Taylor, Robin (1987). "Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds". Journal of the Chemical Society, Perkin Transactions 2 (12): S1–S19. doi:10.1039/P298700000S1. http://pubs.rsc.org/en/content/articlelanding/1987/p2/p298700000s1.
- ↑ Boulatov, Roman, ed (2015). Polymer Mechanochemistry. Springer. p. 9. ISBN 978-3-319-22824-2.
- ↑ Eric V. Anslyn and Dennis A. Dougherty. Modern Physical Organic Chemistry. 2006. pages 850-852
- ↑ S. W. Benson, Thermochemical Kinetics, S. 273, J. Wiley & Sons, New York, London, Sydney, Toronto 1976
- ↑ Dewar, M. J. (1984). "Chemical Implications of σ Conjugation". J. Am. Chem. Soc. 106 (3): 669–682. doi:10.1021/ja00315a036.
- ↑ Cremer, D. (1988). "Pros and Cons of σ-Aromaticity". Tetrahedron 44 (2): 7427–7454. doi:10.1016/s0040-4020(01)86238-4.
- ↑ Wu, Wei; Ma, Ben; Wu, Judy I-Chia; von Ragué, Schleyer; Mo, Yirong (2009). "Is Cyclopropane Really the σ-Aromatic Paradigm?". Chemistry: A European Journal 15 (38): 9730–9736. doi:10.1002/chem.200900586. PMID 19562784.
- ↑ Wollweber, Hartmund (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_289.
- ↑ Gordon, Arnold J. (1967). "Halogenation and olefinic nature of cyclopropane". Journal of Chemical Education 44 (8): 461. doi:10.1021/ed044p461.
- ↑ Advanced organic Chemistry, Reactions, mechanisms and structure 3ed. Jerry March ISBN:0-471-85472-7
External links
 |