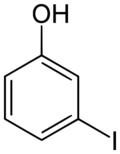
Chemistry:3-Iodophenol
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
3-Iodophenol
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H5IO | |
| Molar mass | 220.009 g·mol−1 |
| Melting point | 118 °C (244 °F; 391 K)[2] |
| Boiling point | 186 °C (367 °F; 459 K)[2] (100 mmHg) |
| Acidity (pKa) | 9.03[1] |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | warning |
| H315, H319, H335 | |
| P261, P305+351+338 | |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
3-Iodophenol (m-iodophenol) is an aromatic organic compound. 3-Iodophenol participates in a variety of coupling reactions in which the iodide substituent is displaced.[4] Well cited examples include thiolate[5] and amine nucleophiles.[6]
3-Iodophenol can be prepared by oxidative decarboxylation of 3-iodobenzoic acid:[7]
- IC
6H
4CO
2H + "O" → IC
6H
4OH + CO
2
References
- ↑ Haynes, p. 5.93
- ↑ 2.0 2.1 Haynes, p. 3.324
- ↑ "3-Iodophenol". Sigma-Aldrich. https://www.sigmaaldrich.com/catalog/product/ALDRICH/I10007.
- ↑ "3-Iodophenol". Fisher Scientific. https://www.fishersci.com/shop/products/3-iodophenol-98-thermo-scientific/AAA1115509.
- ↑ Kwong, Fuk Yee; Buchwald, Stephen L. (2002). "A General, Efficient, and Inexpensive Catalyst System for the Coupling of Aryl Iodides and Thiols". Organic Letters 4 (20): 3517–3520. doi:10.1021/ol0266673. PMID 12323058.
- ↑ Shen, Qilong; Ogata, Tokutaro; Hartwig, John F. (2008). "Highly Reactive, General and Long-Lived Catalysts for Palladium-Catalyzed Amination of Heteroaryl and Aryl Chlorides, Bromides, and Iodides: Scope and Structure–Activity Relationships". Journal of the American Chemical Society 130 (20): 6586–6596. doi:10.1021/ja077074w. PMID 18444639.
- ↑ Xiong, Wenzhang; Shi, Qiu; Liu, Wenbo H. (2022). "Simple and Practical Conversion of Benzoic Acids to Phenols at Room Temperature". Journal of the American Chemical Society 144 (34): 15894–15902. doi:10.1021/jacs.2c07529. PMID 35997485.
Cited sources
- Haynes, William M., ed (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
 |

