Chemistry:10-Hydroxy Lycopodium alkaloids
The 10-hydroxy Lycopodium alkaloids, which include 10-hydroxylycopodine, deacetylpaniculine, and paniculine, are a series of natural products isolated from a Chilean club moss Lycopodium confertum. Deacetylpaniculine and paniculine were also isolated from Lycopodium paniculatum.[1]
The Lycopodium alkaloids are of interest due to their biological activity and unique skeletal characteristics,[2] however, many compounds in this class have not been well studied.[3]
Synthesis
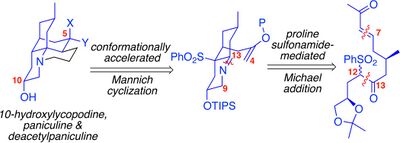
The first enantioselective synthesis of 10-hydroxylycopodine, deacetylpaniculine and paniculine was published by Mrinmoy Saha and Rich Carter in 2013. Two key ring-closure steps were accomplished by an organocatalytic Michael reaction and a Lewis acid-catalyzed Mannich reaction.[4] The impact of the C10 stereochemistry on the Michael addition to construct the C7–C12 bond and on the Mannich cyclization have been explored recently.[5]
See also
- Lycopodium powder
- Lycopodium clavatum
References
- ↑ Muñoz, Orlando M.; Castillo, M.; San Feliciano, A. (January 1990). "High Resolution NMR Studies of Paniculine and Related Lycopodium Compounds". Journal of Natural Products 53 (1): 200–203. doi:10.1021/np50067a032.
- ↑ "Lycopodine and the Lycopodium Alkaloids". http://stoltz.caltech.edu/litmtg/mechclub/2008/AHSC7_08.pdf. dead link needs update
- ↑ Lycopodium Alkaloids: An Intramolecular Michael Reaction Approach |https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0036-1588851
- ↑ Saha, Mrinmoy; Carter, Rich G. (February 2013). "Toward a Unified Approach for the Lycopodines: Synthesis of 10-Hydroxylycopodine, Deacetylpaniculine, and Paniculine". Organic Letters 15 (4): 736–739. doi:10.1021/ol303272w. PMID 23384410.
- ↑ Saha, Mrinmoy; Li, Xin; Collett, Nathan D.; Carter, Rich G. (June 2016). "Unified Synthesis of 10-Oxygenated Lycopodium Alkaloids: Impact of C10-Stereochemistry on Reactivity". J Org Chem 81 (14): 5963–5980. doi:10.1021/acs.joc.6b00900. PMID 27353498.
 |