Biology:Actinopterygii
| Ray-finned fish | |
|---|---|
Error: No valid link was found at the end of line 19. | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Superclass: | Osteichthyes |
| Class: | Actinopterygii Klein, 1885 |
| Subclasses | |
| |
Actinopterygii (/ˌæktɪnɒptəˈrɪdʒiaɪ/; from actino- 'having rays', and grc πτέρυξ (ptérux) 'wing, fins'), members of which are known as ray-finned fish or actinopterygians, is a class of bony fish[2] that comprise over 50% of living vertebrate species.[3] They are so called because of their lightly built fins made of webbings of skin supported by radially extended thin bony spines called lepidotrichia, as opposed to the bulkier, fleshy lobed fins of the sister class Sarcopterygii (lobe-finned fish). Resembling folding fans, the actinopterygian fins can easily change shape and wetted area, providing superior thrust-to-weight ratios per movement compared to sarcopterygian and chondrichthyian fins. The fin rays attach directly to the proximal or basal skeletal elements, the radials, which represent the articulation between these fins and the internal skeleton (e.g., pelvic and pectoral girdles).
The vast majority (~99%) of actinopterygians are teleosts. By species count, they dominate the subphylum Vertebrata, and constitute nearly 99% of the over 30,000 extant species of fish.[4] They are the most abundant nektonic aquatic animals and are ubiquitous throughout freshwater and marine environments from the deep sea to subterranean waters to the highest mountain streams. Extant species can range in size from Paedocypris, at 8 mm (0.3 in); to the massive ocean sunfish, at 2,300 kg (5,070 lb); and to the giant oarfish, at 11 m (36 ft). The largest ever known ray-finned fish, the extinct Leedsichthys from the Jurassic, has been estimated to have grown to 16.5 m (54 ft).
Characteristics

Ray-finned fishes occur in many variant forms. The main features of typical ray-finned fish are shown in the adjacent diagram.
The swim bladder is a more derived structure and used for buoyancy.[5] Except from the bichirs, which just like the lungs of lobe-finned fish have retained the ancestral condition of ventral budding from the foregut, the swim bladder in ray-finned fishes derives from a dorsal bud above the foregut.[6][5] In early forms the swim bladder could still be used for breathing, a trait still present in Holostei (bowfins and gars).[7] In some fish like the arapaima, the swim bladder has been modified for breathing air again,[8] and in other lineages it have been completely lost.[9]
Ray-finned fishes have many different types of scales; but all teleosts have leptoid scales. The outer part of these scales fan out with bony ridges, while the inner part is crossed with fibrous connective tissue. Leptoid scales are thinner and more transparent than other types of scales, and lack the hardened enamel- or dentine-like layers found in the scales of many other fish. Unlike ganoid scales, which are found in non-teleost actinopterygians, new scales are added in concentric layers as the fish grows.[10]
Teleosts also differ from other ray-finned fishes in having gone through a whole-genome duplication (paleopolyploidy).[11][12]
Body shapes and fin arrangements
Ray-finned fish vary in size and shape, in their feeding specializations, and in the number and arrangement of their ray-fins.
Tuna are streamlined for straight line speed with a deeply forked tail
The swordfish is even faster and more streamlined than the tuna
Cod have three dorsal and two anal fins, which give them great maneuverability
Flatfish have developed partially symmetric dorsal and pelvic fins
The four-eyed fish Anableps anableps can see both below and above the water surface
Fangtooth are indifferent swimmers who try to ambush their prey
The first spine of the dorsal fin of anglerfish is modified like a fishing rod with a lure
Bichirs are the most basal living ray-fins; they possess lungs
European conger are ray-finned fish
The benthic batfish Ogcocephalus notatus
The sturgeon Acipenser oxyrhynchus has a cartilaginous endoskeleton
The ambush predator needlefish Belone belone
Seahorses are in the extended pipefish family
The "flying fish" Exocoetus obtusirostris has specialized pectoral fins for gliding
The Jurassic †Leedsichthys was a filter-feeder and the largest ray-finned fish to have ever lived
Lactoria fornasini is a poisonous species of boxfish
Reproduction

In nearly all ray-finned fish, the sexes are separate, and in most species the females spawn eggs that are fertilized externally, typically with the male inseminating the eggs after they are laid. Development then proceeds with a free-swimming larval stage.[13] However other patterns of ontogeny exist, with one of the commonest being sequential hermaphroditism. In most cases this involves protogyny, fish starting life as females and converting to males at some stage, triggered by some internal or external factor. Protandry, where a fish converts from male to female, is much less common than protogyny.[14]
Most families use external rather than internal fertilization.[15] Of the oviparous teleosts, most (79%) do not provide parental care.[16] Viviparity, ovoviviparity, or some form of parental care for eggs, whether by the male, the female, or both parents is seen in a significant fraction (21%) of the 422 teleost families; no care is likely the ancestral condition.[16] The oldest case of viviparity in ray-finned fish is found in Middle Triassic species of †Saurichthys.[17] Viviparity is relatively rare and is found in about 6% of living teleost species; male care is far more common than female care.[16][18] Male territoriality "preadapts" a species for evolving male parental care.[19][20]
There are a few examples of fish that self-fertilise. The mangrove rivulus is an amphibious, simultaneous hermaphrodite, producing both eggs and spawn and having internal fertilisation. This mode of reproduction may be related to the fish's habit of spending long periods out of water in the mangrove forests it inhabits. Males are occasionally produced at temperatures below 19 °C (66 °F) and can fertilise eggs that are then spawned by the female. This maintains genetic variability in a species that is otherwise highly inbred.[21]
Classification and fossil record
Actinopterygii is divided into the classes Cladistia and Actinopteri. The latter comprises the subclasses Chondrostei and Neopterygii. The Neopterygii, in turn, is divided into the infraclasses Holostei and Teleostei. During the Mesozoic (Triassic, Jurassic, Cretaceous) and Cenozoic the teleosts in particular diversified widely. As a result, 96% of living fish species are teleosts (40% of all fish species belong to the teleost subgroup Acanthomorpha), while all other groups of actinopterygians represent depauperate lineages.[22]
The classification of ray-finned fishes can be summarized as follows:
- Cladistia, which include bichirs and reedfish
- Actinopteri, which include:
- Chondrostei, which include Acipenseriformes (paddlefishes and sturgeons)
- Neopterygii, which include:
- Teleostei (most living fishes)
- Holostei, which include:
- Lepisosteiformes (gars)
- Amiiformes (bowfin)
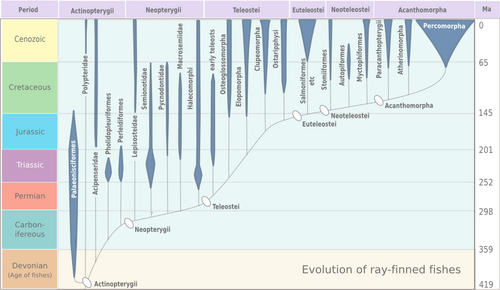
The cladogram below shows the main clades of living actinopterygians and their evolutionary relationships to other extant groups of fishes and the four-limbed vertebrates (tetrapods).[23][24] The latter include mostly terrestrial species but also groups that became secondarily aquatic (e.g. Whales and Dolphins). Tetrapods evolved from a group of bony fish during the Devonian period.[25] Approximate divergence dates for the different actinopterygian clades (in millions of years, mya) are from Near et al., 2012.[23]
Lua error: Internal error: The interpreter exited with status 1.
The polypterids (bichirs and reedfish) are the sister lineage of all other actinopterygians, the Acipenseriformes (sturgeons and paddlefishes) are the sister lineage of Neopterygii, and Holostei (bowfin and gars) are the sister lineage of teleosts. The Elopomorpha (eels and tarpons) appear to be the most basal teleosts.[23]
The earliest known fossil actinopterygian is Andreolepis hedei, dating back 420 million years (Late Silurian), remains of which have been found in Russia , Sweden, and Estonia.[26] Crown group actinopterygians most likely originated near the Devonian-Carboniferous boundary.[27] The earliest fossil relatives of modern teleosts are from the Triassic period (Prohalecites, Pholidophorus),[28][29] although it is suspected that teleosts originated already during the Paleozoic Era.[23]
| Chondrostei | Chondrostei (cartilage bone) is a subclass of primarily cartilaginous fish showing some ossification. Earlier definitions of Chondrostei are now known to be paraphyletic, meaning that this subclass does not contain all the descendants of their common ancestor. There used to be 52 species divided among two orders, the Acipenseriformes (sturgeons and paddlefishes) and the Polypteriformes (reedfishes and bichirs). Reedfish and birchirs are now separated from the Chondrostei into their own sister lineage, the Cladistia. It is thought that the chondrosteans evolved from bony fish but lost the bony hardening of their cartilaginous skeletons, resulting in a lightening of the frame. Elderly chondrosteans show beginnings of ossification of the skeleton, suggesting that this process is delayed rather than lost in these fish.[30] This group had once been classified with the sharks: the similarities are obvious, as not only do the chondrosteans mostly lack bone, but the structure of the jaw is more akin to that of sharks than other bony fish, and both lack scales (excluding the Polypteriforms). Additional shared features include spiracles and, in sturgeons, a heterocercal tail (the vertebrae extend into the larger lobe of the caudal fin). However the fossil record suggests that these fish have more in common with the Teleostei than their external appearance might suggest.[30] | |
|---|---|---|
| Neopterygii |






Taxonomy
The listing below is a summary of all extinct (indicated by a dagger, †) and living groups of Actinopterygii with their respective taxonomic rank. The taxonomy follows Phylogenetic Classification of Bony Fishes[24][31] with notes when this differs from Nelson,[3] ITIS[32] and FishBase[33] and extinct groups from Van der Laan 2016[34] and Xu 2021.[35]
- Order †?Asarotiformes Schaeffer 1968
- Order †?Discordichthyiformes Minikh 1998
- Order †?Paphosisciformes Grogan & Lund 2015
- Order †?Scanilepiformes Selezneya 1985
- Order †Cheirolepidiformes Kazantseva-Selezneva 1977
- Order †Paramblypteriformes Heyler 1969
- Order †Rhadinichthyiformes
- Order †Palaeonisciformes Hay 1902
- Order †Tarrasiiformes sensu Lund & Poplin 2002
- Order †Ptycholepiformes Andrews et al. 1967
- Order †Haplolepidiformes Westoll 1944
- Order †Aeduelliformes Heyler 1969
- Order †Platysomiformes Aldinger 1937
- Order †Dorypteriformes Cope 1871
- Order †Eurynotiformes Sallan & Coates 2013
- Class Cladistia Pander 1860
- Order †Guildayichthyiformes Lund 2000
- Order Polypteriformes Bleeker 1859 (bichirs and reedfishes)[36]
- Class Actinopteri Cope 1972 s.s.
- Order †Elonichthyiformes Kazantseva-Selezneva 1977
- Order †Phanerorhynchiformes
- Order †Bobasatraniiformes Berg 1940
- Order †Saurichthyiformes Aldinger 1937
- Subclass Chondrostei Müller, 1844
- Order †Birgeriiformes Heyler 1969
- Order †Chondrosteiformes Aldinger, 1937
- Order Acipenseriformes Berg 1940 (includes sturgeons and paddlefishes)
- Subclass Neopterygii Regan 1923 sensu Xu & Wu 2012
- Order †Pholidopleuriformes Berg 1937
- Order †Redfieldiiformes Berg 1940
- Order †Platysiagiformes Brough 1939
- Order †Polzbergiiformes Griffith 1977
- Order †Perleidiformes Berg 1937
- Order †Louwoichthyiformes Xu 2021
- Order †Peltopleuriformes Lehman 1966
- Order †Luganoiiformes Lehman 1958
- Order †Pycnodontiformes Berg 1937
- Infraclass Holostei Müller 1844
- Division Halecomorphi Cope 1872 sensu Grande & Bemis 1998
- Order †Parasemionotiformes Lehman 1966
- Order †Ionoscopiformes Grande & Bemis 1998
- Order Amiiformes Huxley 1861 sensu Grande & Bemis 1998 (bowfins)
- Division Ginglymodi Cope 1871
- Order †Dapediiformes Thies & Waschkewitz 2015
- Order †Semionotiformes Arambourg & Bertin 1958
- Order Lepisosteiformes Hay 1929 (gars)
- Division Halecomorphi Cope 1872 sensu Grande & Bemis 1998
- Clade Teleosteomorpha Arratia 2000 sensu Arratia 2013
- Order †Prohaleciteiformes Arratia 2017
- Division Aspidorhynchei Nelson, Grand & Wilson 2016
- Order †Aspidorhynchiformes Bleeker 1859
- Order †Pachycormiformes Berg 1937
- Infraclass Teleostei Müller 1844 sensu Arratia 2013
- Order †?Araripichthyiformes
- Order †?Ligulelliiformes Taverne 2011
- Order †?Tselfatiiformes Nelson 1994
- Order †Pholidophoriformes Berg 1940
- Order †Dorsetichthyiformes Nelson, Grand & Wilson 2016
- Order †Leptolepidiformes
- Order †Crossognathiformes Taverne 1989
- Order †Ichthyodectiformes Bardeck & Sprinkle 1969
- Teleocephala de Pinna 1996 s.s.
- Megacohort Elopocephalai Patterson 1977 sensu Arratia 1999 (Elopomorpha Greenwood et al. 1966)
- Order Elopiformes Gosline 1960 (ladyfishes and tarpon)
- Order Albuliformes Greenwood et al. 1966 sensu Forey et al. 1996 (bonefishes)
- Order Notacanthiformes Goodrich 1909 (halosaurs and spiny eels)
- Order Anguilliformes Jarocki 1822 sensu Goodrich 1909 (true eels)
- Megacohort Osteoglossocephalai sensu Arratia 1999
- Supercohort Osteoglossocephala sensu Arratia 1999 (Osteoglossomorpha Greenwood et al. 1966)
- Order †Lycopteriformes Chang & Chou 1977
- Order Hiodontiformes McAllister 1968 sensu Taverne 1979 (mooneye and goldeye)
- Order Osteoglossiformes Regan 1909 sensu Zhang 2004 (bony-tongued fishes)
- Supercohort Clupeocephala Patterson & Rosen 1977 sensu Arratia 2010
- Cohort Otomorpha Wiley & Johnson 2010 (Otocephala; Ostarioclupeomorpha)
- Subcohort Clupei Wiley & Johnson 2010 (Clupeomorpha Greenwood et al. 1966)
- Order †Ellimmichthyiformes Grande 1982
- Order Clupeiformes Bleeker 1859 (herrings and anchovies)
- Subcohort Alepocephali
- Order Alepocephaliformes Marshall 1962
- Subcohort Ostariophysi Sagemehl 1885
- Section Anotophysa (Rosen & Greenwood 1970) Sagemehl 1885
- Order †Sorbininardiformes Taverne 1999
- Order Gonorynchiformes Regan 1909 (milkfishes)
- Section Otophysa Garstang 1931
- Order Cypriniformes Bleeker 1859 sensu Goodrich 1909 (barbs, carp, danios, goldfishes, loaches, minnows, rasboras)
- Order Characiformes Goodrich 1909 (characins, pencilfishes, hatchetfishes, piranhas, tetras, dourado / golden (genus Salminus) and pacu)
- Order Gymnotiformes Berg 1940 (electric eels and knifefishes)
- Order Siluriformes Cuvier 1817 sensu Hay 1929 (catfishes)
- Section Anotophysa (Rosen & Greenwood 1970) Sagemehl 1885
- Subcohort Clupei Wiley & Johnson 2010 (Clupeomorpha Greenwood et al. 1966)
- Cohort Euteleosteomorpha (Greenwood et al. 1966) (Euteleostei Greenwood 1967 sensu Johnson & Patterson 1996)
- Subcohort Lepidogalaxii
- Lepidogalaxiiformes Betancur-Rodriguez et al. 2013 (salamanderfish)
- Subcohort Protacanthopterygii Greenwood et al. 1966 sensu Johnson & Patterson 1996
- Order Argentiniformes (barreleyes and slickheads) (formerly in Osmeriformes)
- Order Galaxiiformes
- Order Salmoniformes Bleeker 1859 sensu Nelson 1994 (salmon and trout)
- Order Esociformes Bleeker 1859 (pike)
- Subcohort Stomiati
- Order Osmeriformes (smelts)
- Order Stomiatiformes Regan 1909 (bristlemouths and marine hatchetfishes)
- Subcohort Neoteleostei Nelson 1969
- Infracohort Ateleopodia
- Order Ateleopodiformes (jellynose fish)
- Infracohort Eurypterygia Rosen 1973
- Section Aulopa [Cyclosquamata Rosen 1973]
- Order Aulopiformes Rosen 1973 (Bombay duck and lancetfishes)
- Section Ctenosquamata Rosen 1973
- Subsection Myctophata [Scopelomorpha]
- Order Myctophiformes Regan 1911 (lanternfishes)
- Subsection Acanthomorpha Betancur-Rodriguez et al. 2013
- Division Lampridacea Betancur-Rodriguez et al. 2013 [Lampridomorpha; Lampripterygii]
- Order Lampriformes Regan 1909 (oarfish, opah and ribbonfishes)
- Division Paracanthomorphacea sensu Grande et al. 2013 (Paracanthopterygii Greenwood 1937)
- Order Percopsiformes Berg 1937 (cavefishes and trout-perches)
- Order †Sphenocephaliformes Rosen & Patterson 1969
- Order Zeiformes Regan 1909 (dories)
- Order Stylephoriformes Miya et al. 2007
- Order Gadiformes Goodrich 1909 (cods)
- Division Polymixiacea Betancur-Rodriguez et al. 2013 (Polymyxiomorpha; Polymixiipterygii)
- Order †Pattersonichthyiformes Gaudant 1976
- Order †Ctenothrissiformes Berg 1937
- Order Polymixiiformes Lowe 1838 (beardfishes)
- Division Euacanthomorphacea Betancur-Rodriguez et al. 2013 (Euacanthomorpha sensu Johnson & Patterson 1993; Acanthopterygii Gouan 1770 sensu])
- Subdivision Berycimorphaceae Betancur-Rodriguez et al. 2013
- Order Beryciformes (fangtooths and pineconefishes) (incl. Stephanoberyciformes; Cetomimiformes)
- Subdivision Holocentrimorphaceae Betancur-Rodriguez et al. 2013
- Order Holocentriformes (Soldierfishes)
- Subdivision Percomorphaceae Betancur-Rodriguez et al. 2013 (Percomorpha sensu Miya et al. 2003; Acanthopteri)
- Series Ophidiimopharia Betancur-Rodriguez et al. 2013
- Order Ophidiiformes (pearlfishes)
- Series Batrachoidimopharia Betancur-Rodriguez et al. 2013
- Order Batrachoidiformes (toadfishes)
- Series Gobiomopharia Betancur-Rodriguez et al. 2013
- Order Kurtiformes(Nurseryfishes and cardinalfishes)
- Order Gobiiformes(Sleepers and gobies)
- Series Scombrimopharia Betancur-Rodriguez et al. 2013
- Order Syngnathiformes (seahorses, pipefishes, sea moths, cornetfishes and flying gurnards[37])
- Order Scombriformes (Tunas and (mackerels)
- Series Carangimopharia Betancur-Rodriguez et al. 2013
- Subseries Anabantaria Betancur-Rodriguez et al. 2014
- Order Synbranchiformes (swamp eels)
- Order Anabantiformes (Labyrinthici) (gouramies, snakeheads, )
- Subseries Carangaria Betancur-Rodriguez et al. 2014
- Carangaria incertae sedis
- Order Istiophoriformes Betancur-Rodriguez 2013 (Marlins, swordfishes, billfishes)
- Order Carangiformes (Jack mackerels, pompanos)
- Order Pleuronectiformes Bleeker 1859 (flatfishes)
- Subseries Ovalentaria Smith & Near 2012 (Stiassnyiformes sensu Li et al. 2009)
- Ovalentaria incertae sedis
- Order Cichliformes Betancur-Rodriguez et al. 2013 (Cichlids, Convict blenny, leaf fishes)
- Order Atheriniformes Rosen 1964 (silversides and rainbowfishes)
- Order Cyprinodontiformes Berg 1940 (livebearers, killifishes)
- Order Beloniformes Berg 1940 (flyingfishes and ricefishes)
- Order Mugiliformes Berg 1940 (mullets)
- Order Blenniiformes Springer 1993 (Blennies)
- Order Gobiesociformes Gill 1872 (Clingfishes)
- Subseries Anabantaria Betancur-Rodriguez et al. 2014
- Series Eupercaria Betancur-Rodriguez et al. 2014 (Percomorpharia Betancur-Rodriguez et al. 2013)
- Eupercaria incertae sedis
- Order Gerreiformes (Mojarras)
- Order Labriformes (Wrasses and Parrotfishes)
- Order Caproiformes (Boarfishes)
- Order Lophiiformes Garman 1899 (Anglerfishes)
- Order Tetraodontiformes Regan 1929 (Filefishes and pufferfish)
- Order Centrarchiformes Bleeker 1859 (Sunfishes and mandarin fishes)
- Order Gasterosteiformes (Sticklebacks and relatives)
- Order Scorpaeniformes (Lionfishes and relatives)
- Order Perciformes Bleeker 1859
- Series Ophidiimopharia Betancur-Rodriguez et al. 2013
- Subdivision Berycimorphaceae Betancur-Rodriguez et al. 2013
- Division Lampridacea Betancur-Rodriguez et al. 2013 [Lampridomorpha; Lampripterygii]
- Subsection Myctophata [Scopelomorpha]
- Section Aulopa [Cyclosquamata Rosen 1973]
- Infracohort Ateleopodia
- Subcohort Lepidogalaxii
- Cohort Otomorpha Wiley & Johnson 2010 (Otocephala; Ostarioclupeomorpha)
- Supercohort Osteoglossocephala sensu Arratia 1999 (Osteoglossomorpha Greenwood et al. 1966)
- Megacohort Elopocephalai Patterson 1977 sensu Arratia 1999 (Elopomorpha Greenwood et al. 1966)
References
- ↑ Zhao, W.; Zhang, X.; Jia, G.; Shen, Y.; Zhu, M. (2021). "The Silurian-Devonian boundary in East Yunnan (South China) and the minimum constraint for the lungfish-tetrapod split". Science China Earth Sciences 64 (10): 1784–1797. doi:10.1007/s11430-020-9794-8. Bibcode: 2021ScChD..64.1784Z. https://www.researchgate.net/publication/353479392.
- ↑ Kardong, Kenneth (2015). Vertebrates: Comparative Anatomy, Function, Evolution. New York: McGraw-Hill Education. pp. 99–100. ISBN 978-0-07-802302-6.
- ↑ 3.0 3.1 Nelson, Joseph S. (2016). Fishes of the World. John Wiley & Sons. ISBN 978-1-118-34233-6.
- ↑ (Davis, Brian 2010).
- ↑ 5.0 5.1 Funk, Emily; Breen, Catriona; Sanketi, Bhargav; Kurpios, Natasza; McCune, Amy (2020). "Changing in Nkx2.1, Sox2, Bmp4, and Bmp16 expression underlying the lung-to-gas bladder evolutionary transition in ray-finned fishes". Evolution & Development 22 (5): 384–402. doi:10.1111/ede.12354. PMID 33463017.
- ↑ Funk, Emily C.; Breen, Catriona; Sanketi, Bhargav D.; Kurpios, Natasza; McCune, Amy (25 September 2020). "Changes in Nkx2.1, Sox2, Bmp4, and Bmp16 expression underlying the lung-to-gas bladder evolutionary transition in ray-finned fishes". Evolution & Development 22 (5): 384–402. doi:10.1111/ede.12354. PMID 33463017.
- ↑ Zhang, Ruihua; Liu, Qun; Pan, Shanshan; Zhang, Yingying; Qin, Yating; Du, Xiao; Yuan, Zengbao; Lu, Yongrui et al. (13 September 2023). "A single-cell atlas of West African lungfish respiratory system reveals evolutionary adaptations to terrestrialization". Nature Communications 14 (1): 5630. doi:10.1038/s41467-023-41309-3. PMID 37699889. Bibcode: 2023NatCo..14.5630Z.
- ↑ Scadeng, Miriam; McKenzie, Christina; He, Weston; Bartsch, Hauke; Dubowitz, David J.; Stec, Dominik; St. Leger, Judy (25 November 2020). "Morphology of the Amazonian Teleost Genus Arapaima Using Advanced 3D Imaging". Frontiers in Physiology 11: 260. doi:10.3389/fphys.2020.00260. PMID 32395105.
- ↑ Martin, Rene P; Dias, Abigail S; Summers, Adam P; Gerringer, Mackenzie E (16 October 2022). "Bone Density Variation in Rattails (Macrouridae, Gadiformes): Buoyancy, Depth, Body Size, and Feeding". Integrative Organismal Biology 4 (1): obac044. doi:10.1093/iob/obac044. PMID 36381998.
- ↑ "Actinopterygii Klein, 1885" (in en). https://www.gbif.org/species/113225725.
- ↑ Davesne, Donald; Friedman, Matt; Schmitt, Armin D.; Fernandez, Vincent; Carnevale, Giorgio; Ahlberg, Per E.; Sanchez, Sophie; Benson, Roger B. J. (27 July 2021). "Fossilized cell structures identify an ancient origin for the teleost whole-genome duplication". Proceedings of the National Academy of Sciences 118 (30). doi:10.1073/pnas.2101780118. PMID 34301898. Bibcode: 2021PNAS..11801780D.
- ↑ Parey, Elise; Louis, Alexandra; Montfort, Jerome; Guiguen, Yann; Crollius, Hugues Roest; Berthelot, Camille (12 August 2022). "An atlas of fish genome evolution reveals delayed rediploidization following the teleost whole-genome duplication". Genome Research 32 (9): 1685–1697. doi:10.1101/gr.276953.122. PMID 35961774. PMC 9528989. https://genome.cshlp.org/content/early/2022/08/12/gr.276953.122.
- ↑ Dorit, R.L.; Walker, W.F.; Barnes, R.D. (1991). Zoology. Saunders College Publishing. p. 819. ISBN 978-0-03-030504-7. https://archive.org/details/zoology0000dori.
- ↑ Avise, J.C.; Mank, J.E. (2009). "Evolutionary perspectives on hermaphroditism in fishes". Sexual Development 3 (2–3): 152–163. doi:10.1159/000223079. PMID 19684459. https://escholarship.org/uc/item/1px4b8qn.
- ↑ Pitcher, T (1993). The Behavior of Teleost Fishes. London: Chapman & Hall.
- ↑ 16.0 16.1 16.2 Reynolds, John; Nicholas B. Goodwin; Robert P. Freckleton (19 March 2002). "Evolutionary Transitions in Parental Care and Live Bearing in Vertebrates". Philosophical Transactions of the Royal Society B: Biological Sciences 357 (1419): 269–281. doi:10.1098/rstb.2001.0930. PMID 11958696.
- ↑ Maxwell (2018). "Re-evaluation of the ontogeny and reproductive biology of the Triassic fish Saurichthys (Actinopterygii, Saurichthyidae)". Palaeontology 61: 559–574. doi:10.5061/dryad.vc8h5.
- ↑ Clutton-Brock, T. H. (1991). The Evolution of Parental Care. Princeton, NJ: Princeton UP.
- ↑ Werren, John; Mart R. Gross; Richard Shine (1980). "Paternity and the evolution of male parentage". Journal of Theoretical Biology 82 (4): 619–631. doi:10.1016/0022-5193(80)90182-4. PMID 7382520. https://www.researchgate.net/publication/222458526. Retrieved 15 September 2013.
- ↑ Baylis, Jeffrey (1981). "The Evolution of Parental Care in Fishes, with reference to Darwin's rule of male sexual selection". Environmental Biology of Fishes 6 (2): 223–251. doi:10.1007/BF00002788. Bibcode: 1981EnvBF...6..223B.
- ↑ Wootton, Robert J.; Smith, Carl (2014). Reproductive Biology of Teleost Fishes. Wiley. ISBN 978-1-118-89139-1. https://books.google.com/books?id=_YnjBAAAQBAJ.
- ↑ Sallan, Lauren C. (February 2014). "Major issues in the origins of ray-finned fish (Actinopterygii) biodiversity". Biological Reviews 89 (4): 950–971. doi:10.1111/brv.12086. PMID 24612207.
- ↑ 23.0 23.1 23.2 23.3 Thomas J. Near (2012). "Resolution of ray-finned fish phylogeny and timing of diversification". PNAS 109 (34): 13698–13703. doi:10.1073/pnas.1206625109. PMID 22869754. Bibcode: 2012PNAS..10913698N.
- ↑ 24.0 24.1 Betancur-R, Ricardo (2013). "The Tree of Life and a New Classification of Bony Fishes". PLOS Currents Tree of Life 5 (Edition 1). doi:10.1371/currents.tol.53ba26640df0ccaee75bb165c8c26288. PMID 23653398.
- ↑ Laurin, M.; Reisz, R.R. (1995). "A reevaluation of early amniote phylogeny". Zoological Journal of the Linnean Society 113 (2): 165–223. doi:10.1111/j.1096-3642.1995.tb00932.x.
- ↑ "Fossilworks: Andreolepis". http://paleodb.org/cgi-bin/bridge.pl?action=checkTaxonInfo&taxon_no=34968&is_real_user=1.
- ↑ Henderson, Struan; Dunne, Emma M.; Fasey, Sophie A.; Giles, Sam (3 October 2022). "The early diversification of ray-finned fishes (Actinopterygii): hypotheses, challenges and future prospects". Biological Reviews 98 (1): 284–315. doi:10.1111/brv.12907. PMID 36192821.
- ↑ Arratia, G. (2015). "Complexities of early teleostei and the evolution of particular morphological structures through time.". Copeia 103 (4): 999–1025. doi:10.1643/CG-14-184.
- ↑ Romano, Carlo; Koot, Martha B.; Kogan, Ilja; Brayard, Arnaud; Minikh, Alla V.; Brinkmann, Winand; Bucher, Hugo; Kriwet, Jürgen (February 2016). "Permian-Triassic Osteichthyes (bony fishes): diversity dynamics and body size evolution". Biological Reviews 91 (1): 106–147. doi:10.1111/brv.12161. PMID 25431138.
- ↑ 30.0 30.1 "Chondrosteans: Sturgeon Relatives". paleos.com. http://www.palaeos.com/Vertebrates/Units/090Teleostomi/090.300.html.
- ↑ Betancur-Rodriguez (2017). "Phylogenetic Classification of Bony Fishes Version 4". BMC Evolutionary Biology 17 (1): 162. doi:10.1186/s12862-017-0958-3. PMID 28683774.
- ↑ Lua error: Internal error: The interpreter exited with status 1.
- ↑ R. Froese and D. Pauly, ed (February 2006). "FishBase". http://www.fishbase.org.
- ↑ Van der Laan, Richard (2016). Family-group names of fossil fishes. doi:10.13140/RG.2.1.2130.1361. https://www.researchgate.net/publication/317888989.
- ↑ Xu, Guang-Hui (2021-01-09). "A new stem-neopterygian fish from the Middle Triassic (Anisian) of Yunnan, China, with a reassessment of the relationships of early neopterygian clades" (in en). Zoological Journal of the Linnean Society 191 (2): 375–394. doi:10.1093/zoolinnean/zlaa053. ISSN 0024-4082. https://academic.oup.com/zoolinnean/article/191/2/375/5859858.
- ↑ In Nelson, Polypteriformes is placed in its own subclass Cladistia.
- ↑ In Nelson and ITIS, Syngnathiformes is placed as the suborder Syngnathoidei of the order Gasterosteiformes.
Lua error: Internal error: The interpreter exited with status 1.
External links
Lua error: Internal error: The interpreter exited with status 1. Lua error: Internal error: The interpreter exited with status 1.
Wikidata ☰ Q127282 entry
Lua error: Internal error: The interpreter exited with status 1.