Physics:Zwitterion
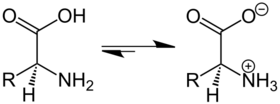
In chemistry, a zwitterion (/ˈtsvɪtəˌraɪən/ TSVIT-ə-rye-ən; from de Zwitter [ˈtsvɪtɐ] 'hermaphrodite'), formerly called a dipolar ion, is a molecule with two or more functional groups, of which at least one has a positive and one has a negative electrical charge and the net charge of the entire molecule is zero. Because they contain at least one positive and one negative charge, zwitterions are also sometimes called inner salts.[1] The charges on the different functional groups balance each other out, and the molecule as a whole is electrically neutral. The pH where this happens is known as the isoelectric point.[2][3]
Unlike simple amphoteric compounds that may only form either a cationic or anionic species, a zwitterion simultaneously has both ionic states.[4]
Examples
Amino acids are examples of zwitterions. These compounds contain an ammonium and a carboxylate group, and can be viewed as arising via a kind of intramolecular acid–base reaction: The amine group deprotonates the carboxylic acid.
- H2NRCHCOOH ⇌ +H3NRCHCOO−
The zwitterionic structure of glycine in the solid state has been confirmed by neutron diffraction measurements.[5] At least in some cases, the zwitterionic form of amino acids also persists in the gas phase.[6]
In addition to the amino acids, many other compounds that contain both acidic and basic centres tautomerize to the zwitterionic form. Examples, such as bicine and tricine, contain a basic secondary or tertiary amine fragment together with a carboxylic acid fragment. Neutron diffraction measurements show that solid sulfamic acid exists as a zwitterion.[7] Many alkaloids, such as psilocybin, exist as zwitterions because they contain carboxylates and ammonium centres.
Many zwitterions contain quaternary ammonium cations. Since it lacks N–H bonds, the ammonium center cannot participate in tautomerization. Zwitterions containing quaternary ammonium centers are common in biology; a common example are the betaines, which serve as electrolytes in fish. The membrane-forming phospholipids are also commonly zwitterions. The polar head groups in these compounds are zwitterions, resulting from the presence of the anionic phosphate and cationic quaternary ammonium centres.[8]
A ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. It is a type of zwitterion.
Calculation of the isoelectric point
The pH value at the isoelectric point can be calculated from the acid dissociation constants of the acidic and basic groups of the zwitterion (except at high dilution):
- [math]\displaystyle{ \ce{pH}=\frac{\ce pK_\ce{a_1}+\ce pK_\ce{a_2}} {2} }[/math]
Derivation:
The acid dissociation constants are defined as:
- [math]\displaystyle{ \begin{align} K_\ce{a_1}=c\left(\ce{H3O+}\right)\cdot\frac{c(\ce{Zwitterion})}{c(\ce{Cation})}\\[10px] K_\ce{a_2}=c\left(\ce{H3O+}\right)\cdot\frac{c(\ce{Anion})}{c(\ce{Zwitterion})} \end{align} }[/math]
Multiplication of these two equations eliminates the concentration of the zwitterion:
- [math]\displaystyle{ K_\ce{a_1}\cdot K_\ce{a_2}=c\left(\ce{H3O+}\right)^2 \cdot\frac{c(\ce{Anion})}{c(\ce{Cation})} }[/math]
Because the concentrations of the anion and cation are equal at the isoelectric point, the equation simplifies to:
- [math]\displaystyle{ K_\ce{a_1}\cdot K_\ce{a_2} = c\left(\ce{H3O+}\right)^2 }[/math]
Taking the square root and logarithm on both sides yields:
- [math]\displaystyle{ \ce{pH}=\frac{\ce pK_\ce{a_1}+\ce pK_\ce{a_2}} {2} }[/math]
Multiprotic systems
In the case of acidic amino acids (e.g. aspartic acid) or basic amino acids (e.g. lysine), the pKa values of the two similar groups are taken into consideration: in the case of amino acids with basic side chains, the side chain and the amino group can both take a positive charge at low pH values, and in the case of amino acids with acidic side chains, the two carboxylic acid groups can both take a negative charge at high pH values. The molecule is therefore neutral halfway between these two pK values, when one of the two similar groups is neutral, so the pK values of the two similar groups are substituted into the equation above[9] and the average is taken,[10] for example for lysine: 8.95 + 10.53/2 = 9.74[11] and for aspartic acid: 2.09 + 3.86/2 = 2.98.[12]
Related compounds
The resonance structures that are used to represent the charge delocalization of dipolar compounds frequently include zwitterions, even when there is not complete separation of charges like with a stable zwitterion.[13] Zwitterionic compounds, on the other hand, have stable, separated unit electrical charges on atoms.[1]
Also related are mesoionic compounds, which are dipolar heterocyclic compounds in which both the negative and the positive charge are delocalized.
References
- ↑ 1.0 1.1 IUPAC Gold Book zwitterionic compounds/zwitterions
- ↑ Das, Debajyoti (1978) (in en). Biochemistry. Academic Publishers. pp. 54. ISBN 9788187504825. https://books.google.com/books?id=Gx0fZ_DF5BoC&pg=PA54.
- ↑ Campbell, Mary; Farrell, Shawn (2007-11-20) (in en). Biochemistry. Cengage Learning. pp. 74. ISBN 0495390410. https://books.google.com/books?id=NYa45_BxgukC&pg=PA74.
- ↑ "Definition of amphoteric" (2014), Chemicool. Retrieved 2016-06-14.
- ↑ Jönsson, P.-G.; Kvick, Å. (1972). "Precision neutron diffraction structure determination of protein and nucleic acid components. III. The crystal and molecular structure of the amino acid α-glycine". Acta Crystallographica Section B 28 (6): 1827–1833. doi:10.1107/S0567740872005096. http://journals.iucr.org/b/issues/1972/06/00/a09113/a09113.pdf.
- ↑ Price, William D.; Jockusch, Rebecca A.; Williams, Evan R. (1997). "Is Arginine a Zwitterion in the Gas Phase?". Journal of the American Chemical Society 119 (49): 11988–11989. doi:10.1021/ja9711627. PMID 16479267.
- ↑ R. L. Sass (April 1960). "A neutron diffraction study on the crystal structure of sulfamic acid". Acta Crystallogr. 13 (4): 320–324. doi:10.1107/S0365110X60000789.
- ↑ Nelson, D. L.; Cox, M. M. Lehninger, Principles of Biochemistry 3rd Ed. Worth Publishing: New York, 2000. ISBN:1-57259-153-6.
- ↑ Reinhard Kuhn: Capillary Electrophoresis: Principles and Practice. Springer Science & Business Media, 2013, ISBN:978-3-642-78058-5, S. 79.
- ↑ Cherng-ju Kim: Advanced Pharmaceutics. CRC Press, 2004, ISBN:978-0-203-49291-8, S. 86–99.
- ↑ W. T. Godbey: An Introduction to Biotechnology. Elsevier, 2014, ISBN:978-1-908-81848-5, S. 15.
- ↑ Raymond Chang: Physical Chemistry for the Biosciences. University Science Books, 2005, ISBN:978-1-891-38933-7, S. 291.
- ↑ IUPAC Gold Book dipolar compounds



