Medicine:DPT vaccine
 Global vaccination coverage- diphtheria-tetanus-pertussis (DTP3) immunization[1] | |
| Combination of | |
|---|---|
| Diphtheria vaccine | Vaccine |
| Pertussis vaccine | Vaccine |
| Tetanus vaccine | Vaccine |
| Clinical data | |
| Trade names | Adacel, Boostrix, Revaxis, others |
| AHFS/Drugs.com | UK Drug Information |
| Routes of administration | Intramuscular injection |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| KEGG | |
| | |
The DPT vaccine or DTP vaccine is a class of combination vaccines against three infectious diseases in humans: diphtheria, pertussis (whooping cough), and tetanus.[7] The vaccine components include diphtheria and tetanus toxoids and either killed whole cells of the bacterium that causes pertussis or pertussis antigens. The term toxoid refers to vaccines which use an inactivated toxin produced by the pathogen which they are targeted against to generate an immune response. In this way, the toxoid vaccine generates an immune response which is targeted against the toxin which is produced by the pathogen and causes disease, rather than a vaccine which is targeted against the pathogen itself.[8] The whole cells or antigens will be depicted as either "DTwP"[9] or "DTaP", where the lower-case "w" indicates whole-cell inactivated pertussis and the lower-case "a" stands for "acellular".[10] In comparison to alternative vaccine types, such as live attenuated vaccines, the DTP vaccine does not contain any live pathogen, but rather uses inactivated toxoid (and for pertussis, either a dead pathogen or pure antigens) to generate an immune response; therefore, there is not a risk of use in populations that are immune compromised since there is not any known risk of causing the disease itself. As a result, the DTP vaccine is considered a safe vaccine to use in anyone and it generates a much more targeted immune response specific for the pathogen of interest.[11]
In the United States, the DPT (whole-cell) vaccine was administered as part of the childhood vaccines recommended by the Centers for Disease Control and Prevention (CDC) until 1996, when the acellular DTaP vaccine was licensed for use.[12]
History
Diphtheria and tetanus toxoids and whole-cell[10] pertussis (DTP; now also "DTwP" to differentiate from the broader class of triple-combination vaccines)[9] vaccination was licensed in 1949.[13] Since the introduction of the combination vaccine, there has been an extensive decline in the incidence of pertussis, or whooping cough, the disease which the vaccine protects against. Additionally, the rates of disease have continued to decline as more extensive immunization strategies have been implemented, including booster doses and increased emphasis on increasing health literacy.[14]
In the 20th century, the advancements in vaccinations helped to reduce the incidence of childhood pertussis and had a dramatically positive effect on the health of populations in the United States.[15] However, in the early 21st century, reported instances of the disease increased 20-fold due to a downturn in the number of immunizations received and resulted in numerous fatalities.[16] During the 21st century, many parents declined to vaccinate their children against pertussis for fear of perceived side effects despite scientific evidence showing vaccines to be highly effective and safe.[16] In 2009, the journal Pediatrics concluded the largest risk among unvaccinated children was not the contraction of side effects, but rather the disease that the vaccination aims to protect against.[16]
DTP vaccines with acelluar pertussis (DTaP; see below) were introduced in the 1990s. The reduced range of antigens causes fewer side effects, but results in a more expensive, shorter-lasting,[17] and possibly less protective vaccine compared to DTwP.[18] High-income countries have mostly switched to DTaP. As of 2023, global production of aP remains limited.[19]
Vaccination rates
In 2016, the CDC reported that 80.4% of children in the US have received four or more DTaP vaccinations by 2 years of life.[20] Vaccination rates for children aged 13–17 with one or more TDaP shots was 90.2% in 2019.[20] Only 43.6% of adults (older than 18) have received a TDaP shot in the last 10 years.[20] The CDC aims to increase vaccination rate among 2-year-olds from 80.4% to 90.0%[21]
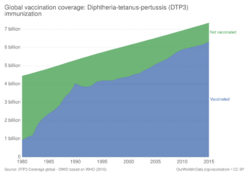
The World Health Organization (WHO) estimates that 89% of people globally have received at least one dose of DTP vaccine and 84% have received three doses of the vaccine, completing the WHO-recommended primary series (DTP3).[22] The WHO also tracks the DTP3 completion rate among one-year-olds on a yearly basis. Yearly DTP3 completion rate is considered a good proxy for the completeness of childhood vaccination in general.[23]
Combination vaccines with acellular pertussis
DTaP and Tdap are both combination vaccines against diphtheria, tetanus, and pertussis. The lower-case "d" and "p" indicate smaller concentrations of diphtheria toxoids and pertussis antigens, and "a" in "ap/aP" indicates that the pertussis toxoids are acellular.[24]
DTaP
DTaP (also DTPa and TDaP) is a combination vaccine against diphtheria, tetanus, and pertussis, in which the pertussis component is acellular.[25] This is in contrast to whole-cell, inactivated DTP (DTwP).[9] The acellular vaccine uses selected antigens of the pertussis pathogen to induce immunity.[17] Because it uses fewer antigens than the whole-cell vaccines, it is considered to cause fewer side effects, but it is also more expensive.[17] Research suggests that the DTP (DTwP) vaccine is more effective than DTaP in conferring immunity, because DTaP's narrower antigen base is less effective against current pathogen strains.[18]
Tdap
Tdap, (also dTpa), is a tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine. It was licensed in the United States for use in adults and adolescents on 10 June 2005.[26] Two Tdap vaccines are available in the US. In January 2011, the US Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) recommended the use of Tdap in adults of all ages, including those age 65 and above.[27] In October 2011, in an effort to reduce the burden of pertussis in infants, the ACIP recommended that unvaccinated pregnant women receive a dose of Tdap. On 24 October 2012, the ACIP voted to recommend the use of Tdap during every pregnancy.[28][29]
The ACIP and Canada's National Advisory Committee on Immunization (NACI) recommended that both adolescents and adults receive Tdap in place of their next Td booster (recommended to be given every ten years).[30][31][32][26] Tdap and Td can be used as prophylaxis for tetanus in wound management. People who will be in contact with young infants are encouraged to get Tdap even if it has been less than five years since Td or TT to reduce the risk of infants being exposed to pertussis. NACI suggests intervals shorter than five years can be used for catch-up programs and other instances where programmatic concerns make five-year intervals difficult.[33]
The WHO recommends a pentavalent vaccine, combining the DTP vaccine with vaccines against Haemophilus influenzae type B and hepatitis B. Evidence on how effective this pentavalent vaccine is compared to the individual vaccines has not yet been determined.[34]
A 2019 study found that state requirements mandating the use of the Tdap vaccine "increased Tdap vaccine take-up and reduced pertussis (whooping cough) incidence by about 32%."[35]
Related combination vaccines
Excluding pertussis
DT and Td vaccines lack the pertussis component.[36][37][38] The Td vaccine is administered to children over the age of seven as well as to adults. It is most commonly administered as a booster shot every 10 years.[36] The Td booster shot may also be administered as protection from a severe burn or dirty wound.[36] The DT vaccine is given to children under the age of seven, who are unable to receive the pertussis antigen in the DTaP vaccine due to a contraindication.[39]
Additional targets
In the United States, a combined inactivated polio (IPV), DTaP, and hepatitis B DTaP-IPV-HepB vaccine is available for children.[40][41] In the UK, all babies born on or after 1 August 2017 are offered a hexavalent vaccine: DTaP, IPV, Haemophilus influenzae, and hepatitis B (DTaP-Hib-HepB-IPV in short).[42]
As of 2023, most of the DTP vaccine procured by UNICEF is of the DTwP-HepB-Hib (pentavalent whole-cell) type. The UNICEF plans to procure the DTwP-HepB-Hib-IPV (hexavalent whole-cell) vaccine starting in 2024.[19]
Contraindications
The DPT vaccine should be avoided in persons who experienced a severe allergic reaction, such as anaphylaxis, to a past vaccine containing tetanus, diphtheria, or pertussis. It should also be avoided in persons with a known severe allergy to an ingredient in the vaccine. If the reaction was caused by tetanus toxoids, the CDC recommends considering a passive immunization with tetanus immune globulin (TIG) if a person has a large or unclean wound.[43] The DPT vaccine should also be avoided if a person developed encephalopathy (seizures, coma, declined consciousness) within seven days of receiving any pertussis-containing vaccine and the encephalopathy cannot be traced to another cause.[44] A DT vaccine is available for children under the ages of seven who have contraindications or precautions to pertussis-containing vaccines.[45]
Side effects
DTaP
Common side effects include soreness where the shot was given, fever, irritability, tenderness, loss of appetite, and vomiting.[25] Most side effects are mild to moderate and may last from one to three days.[25] More serious but rare reactions after a DTaP vaccination may include seizures, lowered consciousness, or a high fever over 105 °F (41 °C).[7] Allergic reactions are uncommon, but are medical emergencies. Signs of an allergic reaction include hives, dyspnea, wheezing, swelling of face and throat, syncope, and tachycardia and the child should be rushed to the nearest hospital.[46]
Tdap
Common side effects include pain or swelling where the shot was given, mild fever, headache, tiredness, nausea, vomiting, diarrhea, and stomach ache.[25] Allergic reactions are possible and have the same presentation and indications as described above for allergic reactions in DTaP. Any individual who has experienced a life-threatening allergic reaction after receiving a previous dose of diphtheria, tetanus, or pertussis containing vaccine should not receive the Tdap vaccination.[25]
In pregnant women, research suggests that Tdap administration may be associated with an increased risk of chorioamnionitis, a placental infection.[47] Increased incidence of fever is also noted in pregnant women.[47] Despite the observed increase in incidence of chorioamnionitis in pregnant women following Tdap administration, there has been no observed increase in the incidence of preterm birth, for which chorioamnionitis is a risk factor.[47][48] Research has not discerned an association between Tdap administration during pregnancy and other serious pregnancy complications such as neonatal death and stillbirth.[47][48] An association between Tdap administration during pregnancy and pregnancy-related hypertensive disorders (such as pre-eclampsia) has not been identified.[48]
Immunization schedules and requirements
France
In France, children are given DTaP-Hib-HepB-IPV vaccines at 2 months (first dose) and 4 months (second dose) with a booster at 11 months.
Netherlands
In the Netherlands, pertussis is known as kinkhoest and DKTP refers to the DTaP-IPV combination vaccine against diphtheria, kinkhoest, tetanus, and polio. DTaP is given as part of the National Immunisation Programme.[49]
United Kingdom
This section is missing information about initial shot. (October 2023) |
In the United Kingdom, Td/IPV[50] is called the "3-in-1 teenage booster" and protects against tetanus, diphtheria and polio. It is given by the NHS to all teenagers aged 14 (the hexavalent vaccine is given to infants and provides the first stage of protection against diphtheria, tetanus, and polio, as well as pertussis, Haemophilus influenzae type B and hepatitis B). Subsequent boosters are recommended for foreign travellers where more than 10 years has passed since their last booster.[51] This is provided on the NHS free of charge due to the significant risk that an imported case of polio could pose to public health in Britain.[52]
United States
The standard immunization regimen for children within the United States is five doses of DTaP between the ages of two months and fifteen years. To be considered fully vaccinated, the Centers for Disease Control and Prevention (CDC) typically requires five doses of Tdap.[53] The CDC recommends that children receive their first dose at two months, the second dose at four months, the third dose at six months, the fourth dose between 15 and 18 months, and the fifth dose between 4–6 years. If the fourth dose of the DTaP immunization regimen falls on or subsequent to the recipient's fourth birthday, the CDC states that only four doses are required to be fully vaccinated.[53] In the instance that an individual under 18 has not received the DTaP vaccine, individuals should be vaccinated on the schedule in accordance with the vaccination "catch up schedule" provided by the CDC.[53]
Infants younger than twelve months of age, specifically less than three months of age, are at highest risk of acquiring pertussis.[54] In U.S., there is no current tetanus-diphtheria-pertussis vaccination (whooping cough) recommended or licensed for new born infants.[54] As a result, in their first few months of life, unprotected infants are at highest risk of life-threatening complications and infections from pertussis. Infants should not receive pertussis vaccination younger than six weeks of age.[55] Ideally, Infants should receive DTaP (name of whooping cough vaccine for children from age 2 months through 6 years) at 2, 4, 6 months of age and they are not protected until the full series is completed.[54] To protect infants younger than twelve months of age not vaccinated with Tdap against pertussis, ACIP also recommends adults (e.g., parents, siblings, grandparents, childcare providers, and healthcare personnel) and children to receive Tdap at least two weeks before being in contact with the infant.[44]
The CDC recommends that adults who have received their childhood DTP series receive a Td or Tdap booster every ten years.[56][57] For adults that have not received the DTP series, the CDC recommends a three-part vaccine series followed by a Td or Tdap booster every ten years.[56]
In pregnancy
According to the CDC's Advisory Committee on Immunization Practices (ACIP) guidelines, one dose of Tdap is recommended during each pregnancy to ensure protection against pertussis in newborn infants.[58] Optimal timing to administer a dose of Tdap during each pregnancy is between 27 through 36 weeks gestation.[58] If Tdap is administered early in pregnancy, it is not recommended to administer again during the 27 through 36 weeks gestation period as only one dose is recommended during pregnancy.[59] In October 2022, Boostrix (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Adsorbed [Tdap]) was approved for immunization during the third trimester of pregnancy to prevent pertussis, commonly known as whooping cough, in infants younger than two months of age.[60]
Pregnant women who have not previously vaccinated with Tdap (i.e., have never received DTP, DTaP, or DT as child or Td or TT as an adult) are recommended to receive a series of three Td vaccinations starting during pregnancy to ensure protection against maternal and neonatal tetanus.[61] In such cases, administration of Tdap is recommended after 20 weeks' gestation,[62][29] and in earlier pregnancy a single dose of Tdap can be substituted for one dose of Td, and then the series completed with Td.[61][29] For pregnant women not previously vaccinated with Tdap, if Tdap is not administered during pregnancy, it should be administered immediately postpartum.[44] Postpartum administration of TDaP is not equivalent to administration of the vaccination during pregnancy.[63] Because the vaccine is administered postpartum, the mother is unable to develop antibodies that can be transferred to the infant in utero, consequently, leaving the infant vulnerable to the diseases preventable by the Tdap Vaccine.[63] Postpartum administration of the TdaP vaccine to the mother seeks to reduce the likelihood that the mother will contract disease that can be subsequently passed on the infant, albeit there will still be a two-week period prior to the protective effects of the vaccine setting in.[63] Postpartum administration is an extension of the concept of "cocooning", a term that refers to the full vaccination of all individuals that may come into direct contact with the infant.[63] Cocooning, like postpartum Tdap administration, is not recommended by the CDC.[63] Cocooning depends on ensuring full vaccination of all individuals that the infant may come into contact with, and there may be financial, administrative or personal barriers that preclude full and timely vaccination of all individuals within the "cocoon".[63]
Brand names
Australia
| Trade name | Approval date | Comments |
|---|---|---|
| Adacel[64] | 2005[65] | Adacel is indicated for active immunisation against tetanus, diphtheria and pertussis in persons aged ten years and over as a booster following primary immunisation[65] and is informally known as 'triple antigen' in Australia.[66] |
| Adacel Polio[67] | 2006[68] | Adacel Polio is indicated for active immunization against diphtheria, tetanus, pertussis and poliomyelitis in adults, adolescents and children aged four years and older as a booster following primary immunization.[68] |
United Kingdom
Brand names in the United Kingdom include Revaxis (Sanofi Pasteur).[69]
United States
(As of January 2020), there are six DTaP vaccines and two Tdap vaccines licensed and available for use in the United States.[70][71] All of them are indicated as childhood vaccinations with the schedules as follows:
| Trade name | Approval date | Comments | Contraindications |
|---|---|---|---|
| Daptacel[72] | 2002[73] | For use in ages six weeks through six years as a five-dose series at 2, 4, and 6 months (6–8 weeks apart) and at 15–20 months of age and at 4–6 years.[72] |
|
| Infanrix[74] | 1997[75] | For use in ages six weeks through six years (before the seventh birthday) as a five-dose series as: a three-dose course at 2, 5, and 6 months (4–8 weeks apart), followed by a two booster doses at 15–20 months of age and 4–6 years of age.[74] |
|
| Kinrix[76] | 2008[77] | DTaP-IPV vaccine; also immunizes against poliomyelitis. Kinrix can be used for the fifth (last) dose in the DTaP immunization series and the fourth dose in the IPV immunization series in children 4–6 years old (before the seventh birthday) whose previous DTaP vaccine doses have been with Infanrix and/or Pediarix for the first three doses and Infanrix for the fourth dose.[76] |
|
| Pediarix[78] | 2002[79] | DTaP-IPV-HepB vaccine; also immunizes against hepatitis B and poliomyelitis as a three-dose series in infants two, four, and six months (4–8 weeks apart).[78] |
|
| Pentacel[80] | 2008[81] | DTaP-IPV/Hib vaccine; also immunizes against invasive Haemophilus influenza type b and poliomyelitis. It is a four-dose series given at: 2, 4, and 6 months, and at 15–18 months of age.[80] |
|
| Quadracel[82] | 2015[83] | DTaP-IPV vaccine; also immunizes against poliomyelitis. It is approved for use as a fifth dose for children aged 4–6 years old in the DTaP vaccination series and as a fourth or fifth dose in the inactivated polio (IPV) series.[82] |
|
| Trade name | Approval date | Comments | Contraindications |
|---|---|---|---|
| Adacel[84] | 2005[85] | For use in ages 10 through 64 as an active booster immunization against tetanus, diphtheria, and pertussis. It may also be administered as prophylaxis for wound management.[84] It has not been shown to be safe or effective as a primary immunization or to complete the series. |
|
| Boostrix[86] | 2005[87] | For use in ages ten and older as a single intramuscular injection into the deltoid as a booster immunization against tetanus, diphtheria, and pertussis. It may also be administered as prophylaxis for wound management.[86] |
|
References
- ↑ "Global vaccination coverage: Diphtheria-tetanus-pertussis (DTP3) immunization". https://ourworldindata.org/grapher/diphtheria-tetanus-pertussis-dtp3-immunization-global.
- ↑ http://www.immunize.org/catg.d/p3073.pdf [bare URL PDF]
- ↑ http://www.immunize.org/catg.d/p3078a.pdf [bare URL PDF]
- ↑ http://www.immunize.org/catg.d/p3078.pdf [bare URL PDF]
- ↑ http://www.immunize.org/catg.d/p3078b.pdf [bare URL PDF]
- ↑ https://www.ema.europa.eu/documents/psusa/diphtheria/tetanus/pertussis-acellular-component-vaccine-adsorbed-diphtheria/tetanus/pertussis-acellular-component-vaccine-adsorbed-reduced-antigens-contents-list-nationally-authorised_en.pdf
- ↑ 7.0 7.1 "DTaP (Diphtheria, Tetanus, Pertussis) Vaccine Information Statement". 1 April 2020. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/dtap.html.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Vaccine Types". 26 April 2021. https://www.hhs.gov/immunization/basics/types/index.html.
- ↑ 9.0 9.1 9.2 "Choosing from Whole Cell and Acellular Pertussis Vaccines-Dilemma for the Developing Countries". Iranian Journal of Public Health 46 (2): 272–273. February 2017. PMID 28451568.
- ↑ 10.0 10.1 "Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP)". MMWR. Recommendations and Reports 67 (2): 1–44. April 2018. doi:10.15585/mmwr.rr6702a1. PMID 29702631.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Understanding Six Types of Vaccine Technologies". https://www.pfizer.com/news/articles/understanding_six_types_of_vaccine_technologies.
- ↑ "Birth-18 Years Immunization Schedule". 2020. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html.
- ↑ "Vaccine Timeline: Historic Dates and Events Related to Vaccines and Immunization". Immunization Action Coalition. 17 May 2013. http://www.immunize.org/timeline.
- ↑ "Pertussis: History of the Disease and Current Prevention Failure". Pulmonary Dysfunction and Disease. Advances in Experimental Medicine and Biology. 934. 2016. pp. 77–82. doi:10.1007/5584_2016_21. ISBN 978-3-319-42009-7.
- ↑ "Lessons learned and applied: what the 20th century vaccine experience can teach us about vaccines in the 21st century". Human Vaccines & Immunotherapeutics 8 (5): 560–568. May 2012. doi:10.4161/hv.19204. PMID 22617834.
- ↑ 16.0 16.1 16.2 "Is Vaccine Refusal Worth The Risk?". NPR. 26 May 2009. https://www.npr.org/templates/story/story.php?storyId=104523437.
- ↑ 17.0 17.1 17.2 "Pertussis Prevention: Reasons for Resurgence, and Differences in the Current Acellular Pertussis Vaccines". Frontiers in Immunology 10: 1344. 2019. doi:10.3389/fimmu.2019.01344. PMID 31333640.
- ↑ 18.0 18.1 "Diphtheria, Tetanus, and Whooping Cough Vaccination". U.S. Centers for Disease Control and Prevention (CDC). 2020. https://www.cdc.gov/vaccines/vpd/dtap-tdap-td/public/index.html.
- ↑ 19.0 19.1 "Diphtheria Tetanus and Pertussis Containing Vaccines: Market and Supply Update". June 2023. https://www.unicef.org/supply/media/17606/file/Diphtheria-Tetanus-Pertussis-Vaccine-Containing-Market-and-Supply-Update-June-2023.pdf.
- ↑ 20.0 20.1 20.2 "FastStats". U.S. Centers for Disease Control and Prevention (CDC). 6 September 2022. https://www.cdc.gov/nchs/fastats/whooping-cough.htm.
- ↑ "Increase the coverage level of 4 doses of the DTaP vaccine in children by age 2 years — IID‑06 - Healthy People 2030 | health.gov". https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-coverage-level-4-doses-dtap-vaccine-children-age-2-years-iid-06.
- ↑ "Diphtheria tetanus toxoid and pertussis (DTP) vaccination coverage". https://immunizationdata.who.int/pages/coverage/dtp.html?CODE=Global&ANTIGEN=&YEAR=.
- ↑ "Prevalence, Trends and Conditions for the DTP3 Vaccine: A 25-Year Historical Perspective". Risk Management and Healthcare Policy 14: 4301–4310. 2021. doi:10.2147/RMHP.S312263. PMID 34703341.
- ↑ "The difference between Tdap and DTaP; dabigatran versus warfarin". JAAPA 24 (1): 14. January 2011. doi:10.1097/01720610-201101000-00002. PMID 21261140.
- ↑ 25.0 25.1 25.2 25.3 25.4 "Safety Information for Diphtheria, Tetanus, and Pertussis Vaccines". 15 June 2020. https://www.cdc.gov/vaccinesafety/vaccines/dtap-tdap-vaccine.html.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 26.0 26.1 "Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel". MMWR. Recommendations and Reports 55 (RR-17): 1–37. December 2006. PMID 17167397. https://www.cdc.gov/mmwr/PDF/rr/rr5517.pdf.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Centers for Disease Control Prevention (CDC) (January 2011). "Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010". MMWR. Morbidity and Mortality Weekly Report 60 (1): 13–15. PMID 21228763. https://www.cdc.gov/mmwr/pdf/wk/mm6001.pdf.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Centers for Disease Control Prevention (CDC) (February 2013). "Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women--Advisory Committee on Immunization Practices (ACIP), 2012". MMWR. Morbidity and Mortality Weekly Report 62 (7): 131–135. PMID 23425962. PMC 4604886. https://www.cdc.gov/mmwr/pdf/wk/mm6207.pdf.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 29.0 29.1 29.2 "Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccines: Updated Recommendations of the Advisory Committee on Immunization Practices — United States, 2019". MMWR. Morbidity and Mortality Weekly Report 69 (3): 77–83. January 2020. doi:10.15585/mmwr.mm6903a5. PMID 31971933.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP)". MMWR. Recommendations and Reports 55 (RR-3): 1–34. March 2006. PMID 16557217. https://www.cdc.gov/mmwr/PDF/rr/rr5503.pdf.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "ACIP Votes to Recommend Use of Combined Tetanus, Diphtheria and Pertussis (Tdap) Vaccine for Adults". https://www.cdc.gov/nip/vaccine/tdap/tdap_adult_recs.pdf.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Interval Between Administration of Vaccines Against Diphtheria, Tetanus, and Pertussis". 14 October 2005. http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/05vol31/acs-dcc-8-9/9_e.html.
- ↑ "General Recommendations on Immunization". U.S. Centers for Disease Control and Prevention (CDC). 2020. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm.
- ↑ "Combined DTP-HBV-HIB vaccine versus separately administered DTP-HBV and HIB vaccines for primary prevention of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae B (HIB)". The Cochrane Database of Systematic Reviews 4 (4): CD005530. April 2012. doi:10.1002/14651858.CD005530.pub3. PMID 22513932.
- ↑ "Direct and Spillover Effects of Middle School Vaccination Requirements". American Economic Journal: Economic Policy 11 (1): 95–125. 2019. doi:10.1257/pol.20170067. ISSN 1945-7731.
- ↑ 36.0 36.1 36.2 "Td (Tetanus, Diphtheria) Vaccine Information Statement". 1 March 2020. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/td.html.
- ↑ "Tetanus and Diphtheria (Td) Vaccine". 13 June 2016. https://www.healthlinkbc.ca/healthlinkbc-files/tetanus-diphtheria-vaccine.
- ↑ "Diphtheria Vaccination". https://www.cdc.gov/vaccines/vpd/diphtheria/index.html.
- ↑ "UpToDate". https://www.uptodate.com/contents/diphtheria-tetanus-and-pertussis-immunization-in-children-7-through-18-years-of-age?search=dtap&source=search_result&selectedTitle=3~60&usage_type=default&display_rank=3.
- ↑ Epidemiology and Prevention of Vaccine-Preventable Diseases (The Pink Book) (10th ed. (2nd printing) ed.). Washington, D.C.: Public Health Foundation. 2008. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/polio-508.pdf. Retrieved 29 November 2008.
- ↑ "Pediarix". U.S. Food and Drug Administration (FDA). 6 November 2019. https://www.fda.gov/vaccines-blood-biologics/vaccines/pediarix.
- ↑ "Hexavalent 6-in-1 vaccine to be made available to newborn babies". https://www.gov.uk/government/news/hexavalent-6-in-1-vaccine-to-be-made-available-to-newborn-babies.
- ↑ "Chapter 21: Tetanus". Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC). 2015. ISBN 978-0990449119. https://www.cdc.gov/vaccines/pubs/pinkbook/tetanus.html.
- ↑ 44.0 44.1 44.2 "Chapter 16: Pertussis". Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC). 2015. ISBN 978-0990449119. https://www.cdc.gov/vaccines/pubs/pinkbook/pert.html.
- ↑ "UpToDate". https://www.uptodate.com/contents/diphtheria-tetanus-and-pertussis-immunization-in-children-6-weeks-through-6-years-of-age.
- ↑ "Diphtheria-Tetanus-Pertussis Vaccine Information Statement | CDC". U.S. Centers for Disease Control and Prevention (CDC). 27 June 2022. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/dtap.html.
- ↑ 47.0 47.1 47.2 47.3 "Safety and effectiveness of acellular pertussis vaccination during pregnancy: a systematic review". BMC Infectious Diseases 20 (1): 136. February 2020. doi:10.1186/s12879-020-4824-3. PMID 32054444.
- ↑ 48.0 48.1 48.2 "Efficacy and safety of pertussis vaccination for pregnant women — a systematic review of randomised controlled trials and observational studies". BMC Pregnancy and Childbirth 17 (1): 390. November 2017. doi:10.1186/s12884-017-1559-2. PMID 29166874.
- ↑ "Dutch National Immunization Program". https://rijksvaccinatieprogramma.nl/english.
- ↑ "3-in-1 teenage booster overview" (in en). 31 July 2019. https://www.nhs.uk/conditions/vaccinations/3-in-1-teenage-booster/.
- ↑ "Tetanus". 18 October 2017. https://www.nhs.uk/conditions/tetanus/.
- ↑ "Travel vaccinations". 23 October 2017. https://www.nhs.uk/conditions/travel-vaccinations/.
- ↑ 53.0 53.1 53.2 "Summary of Pertussis Vaccination Recommendations | CDC". U.S. Centers for Disease Control and Prevention (CDC). 6 May 2022. https://www.cdc.gov/vaccines/vpd/pertussis/recs-summary.html.
- ↑ 54.0 54.1 54.2 "Pertussis | Pregnancy and Whooping Cough | Your Baby Needs Vaccines on Time | CDC". U.S. Centers for Disease Control and Prevention (CDC). 14 February 2019. https://www.cdc.gov/pertussis/pregnant/mom/vaccinate-baby.html.
- ↑ "Protecting infants from pertussis". Canadian Family Physician 60 (2): 138–140. February 2014. PMID 24522676.
- ↑ 56.0 56.1 "Tetanus, diphtheria, and pertussis vaccination". 3 February 2020. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html#note-tdap.
- ↑ "Humoral immunity 10 years after booster immunization with an adolescent and adult formulation combined tetanus, diphtheria, and 5-component acellular pertussis vaccine in the USA". Vaccine 36 (17): 2282–2287. April 2018. doi:10.1016/j.vaccine.2018.03.029. PMID 29573876.
- ↑ 58.0 58.1 "Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccines: Updated Recommendations of the Advisory Committee on Immunization Practices — United States, 2019". MMWR. Morbidity and Mortality Weekly Report 69 (3): 77–83. January 2020. doi:10.15585/mmwr.mm6903a5. PMID 31971933.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Tdap (Pertussis) Vaccine and Pregnancy". 10 April 2019. https://www.cdc.gov/vaccines/pregnancy/hcp-toolkit/tdap-vaccine-pregnancy.html.
- ↑ "FDA Approves Vaccine for Use During Third Trimester of Pregnancy to Prevent Whooping Cough in Infants Younger Than Two Months of Age". U.S. Food and Drug Administration (FDA) (Press release). 7 October 2022. Retrieved 7 October 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 61.0 61.1 Health Care Guideline: Routine Prenatal Care. Fourteenth Edition. By the Institute for Clinical Systems Improvement. July 2010.
- ↑ Centers for Disease Control Prevention (CDC) (October 2011). "Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months --- Advisory Committee on Immunization Practices (ACIP), 2011". MMWR. Morbidity and Mortality Weekly Report 60 (41): 1424–1426. PMID 22012116. https://www.cdc.gov/mmwr/PDF/wk/mm6041.pdf.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 63.0 63.1 63.2 63.3 63.4 63.5 "Tdap (Pertussis) Vaccine and Pregnancy | CDC". U.S. Centers for Disease Control and Prevention (CDC). 15 January 2021. https://www.cdc.gov/vaccines/pregnancy/hcp-toolkit/tdap-vaccine-pregnancy.html.
- ↑ "Adacel". 4 June 2018. https://immunisationhandbook.health.gov.au/vaccines/adacel.
- ↑ 65.0 65.1 "Adacel (Pertussis Vaccine-Acellular Combined with Diphtheria and Tetanus Toxoids (Adsorbed))". 18 July 2020. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2013-PI-01321-1.
- ↑ "Triple Antigen IPV". http://www.stagnessurgery.com.au/triple-antigen-ipv.html.
- ↑ "Adacel Polio". 5 June 2018. https://immunisationhandbook.health.gov.au/vaccines/adacel-polio.
- ↑ 68.0 68.1 "Adacel Polio (Pertussis Vaccine — Acellular and Diphtheria and Tetanus Toxoids (Adsorbed) Combined with Inactivated Poliovirus Type 1, 2 and 3 (Vero cell))". 18 July 2020. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2012-PI-02949-1.
- ↑ NHS https://www.nhs.uk/conditions/vaccinations/3-in-1-teenage-booster/
- ↑ "About Diphtheria, Tetanus, and Pertussis Vaccination". 22 January 2020. https://www.cdc.gov/vaccines/vpd/dtap-tdap-td/hcp/about-vaccine.html.
- ↑ "Licensed Biological Products with Supporting Documents". 7 July 2020. https://www.fda.gov/vaccines-blood-biologics/licensed-biological-products-supporting-documents.
- ↑ 72.0 72.1 72.2 "Daptacel (corynebacterium diphtheriae toxoid antigen (formaldehyde inactivated), clostridium tetani toxoid antigen (formaldehyde inactivated), bordetella pertussis toxoid antigen (glutaraldehyde inactivated), bordetella pertussis filamentous hemagglutinin antigen- formaldehyde inactivated, bordetella pertussis pertactin antigen, and bordetella pertussis fimbriae 2/3 antigen injection, suspension)". Sanofi Pasteur Inc.. 14 May 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=06f34d0f-4e72-41d3-967f-8abf3f2005c1.
- ↑ "Daptacel". 22 July 2017. https://www.fda.gov/vaccines-blood-biologics/vaccines/daptacel.
- ↑ 74.0 74.1 74.2 "Infanrix- diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed suspension". GlaxoSmithKline Biologicals SA. 6 November 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de16dd6a-859b-4180-c6af-f930be14f26a.
- ↑ "Infanrix". 22 July 2017. https://www.fda.gov/vaccines-blood-biologics/vaccines/infanrix.
- ↑ 76.0 76.1 76.2 "Kinrix- diphtheria and tetanus toxoids and acellular pertussis adsorbed and inactivated poliovirus vaccine injection, suspension". GlaxoSmithKline Biologicals SA. 6 November 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6b5f89e9-1292-4f13-5590-44c874bf299c.
- ↑ "Kinrix". 22 July 2017. https://www.fda.gov/vaccines-blood-biologics/vaccines/kinrix.
- ↑ 78.0 78.1 78.2 "Pediarix (diphtheria and tetanus toxoids and acellular pertussis adsorbed, hepatitis b- recombinant and inactivated poliovirus vaccine combined injection, suspension". GlaxoSmithKline Biologicals SA. 6 November 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b63c4c7d-3dbf-419d-84cc-2c1957b92be7.
- ↑ "Pediarix". 23 July 2017. https://www.fda.gov/vaccines-blood-biologics/vaccines/pediarix.
- ↑ 80.0 80.1 80.2 "Pentacel (diphtheria and tetanus toxoids and acellular pertussis adsorbed, inactivated poliovirus and haemophilus b conjugate- tetanus toxoid conjugate vaccine kit)". Sanofi Pasteur Inc.. 5 June 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=377116cf-adfe-40b5-b871-3a0fc8b4103e.
- ↑ "Pentacel". 22 July 2017. https://www.fda.gov/vaccines-blood-biologics/vaccines/pentacel.
- ↑ 82.0 82.1 82.2 "Quadracel- diphtheria and tetanus toxoids and acellular pertussis adsorbed and inactivated poliovirus vaccine injection, suspension". Sanofi Pasteur Inc.. 20 April 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=99d971ad-54da-420d-b025-eb2dab8c4ec9.
- ↑ "Quadracel". 22 July 2017. https://www.fda.gov/vaccines-blood-biologics/vaccines/quadracel.
- ↑ 84.0 84.1 84.2 "Adacel Tdap (clostridium tetani toxoid antigen (formaldehyde inactivated), corynebacterium diphtheriae toxoid antigen (formaldehyde inactivated), bordetella pertussis toxoid antigen (glutaraldehyde inactivated), bordetella pertussis filamentous hemagglutinin antigen- formaldehyde inactivated, bordetella pertussis pertactin antigen, and bordetella pertussis fimbriae 2/3 antigen injection, suspension)". 26 March 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a41b7601-34f2-4a88-a406-f53011fb7de1.
- ↑ "Adacel". https://www.fda.gov/vaccines-blood-biologics/vaccines/adacel.
- ↑ 86.0 86.1 86.2 "Boostrix- tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine, adsorbed suspension". 25 April 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cd98bff9-4602-4268-d68d-029a14a5513b.
- ↑ "Boostrix". https://www.fda.gov/vaccines-blood-biologics/vaccines/boostrix.
Further reading
Diphtheria
- The immunological basis for immunization : module 2: diphtheria — update 2009. World Health Organization (WHO). 2009. ISBN 9789241597869.
- "Chapter 15: Diphtheria". Immunisation against infectious disease. Public Health England. 2013. https://www.gov.uk/government/publications/diphtheria-the-green-book-chapter-15.
- Manual for the surveillance of vaccine-preventable diseases. Atlanta GA: U.S. Centers for Disease Control and Prevention (CDC). March 2019. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt01-dip.html.
Pertussis
- The immunological basis for immunization series: module 4: pertussis, update 2017. World Health Organization (WHO). 2017. ISBN 9789241513173.
- "Chapter 24: Pertussis". Immunisation against infectious disease. Public Health England. 2013. https://www.gov.uk/government/publications/pertussis-the-green-book-chapter-24.
- "Chapter 16: Pertussis". Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC). 2015. ISBN 978-0990449119. https://www.cdc.gov/vaccines/pubs/pinkbook/pert.html.
- "Chapter 10: Pertussis". Manual for the surveillance of vaccine-preventable diseases. Atlanta GA: U.S. Centers for Disease Control and Prevention (CDC). March 2019. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt10-pertussis.html.
Tetanus
- The immunological basis for immunization series: module 3: tetanus: update 2018. World Health Organization (WHO). 2018. ISBN 9789241513616.
- "Chapter 30: Tetanus". Immunisation against infectious disease. Public Health England. 2013. https://www.gov.uk/government/publications/tetanus-the-green-book-chapter-30.
- "Chapter 21: Tetanus". Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC). 2015. ISBN 978-0990449119. https://www.cdc.gov/vaccines/pubs/pinkbook/tetanus.html.
- "Chapter 16: Tetanus". Manual for the surveillance of vaccine-preventable diseases. Atlanta GA: U.S. Centers for Disease Control and Prevention (CDC). March 2019. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt16-tetanus.html.
External links
- "Tdap — Tetanus-Diphtheria-Pertussis Vaccine Information Statement". August 2021. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/tdap.html.
- "DTaP (Diphtheria, Tetanus, Pertussis) Vaccine Information Statement". August 2021. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/dtap.html.
- "DTaP/Tdap/Td ACIP Vaccine Recommendations". 28 January 2020. https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/dtap.html.
- Tetanus Toxoid at the US National Library of Medicine Medical Subject Headings (MeSH)
- Diphtheria-Tetanus Vaccine at the US National Library of Medicine Medical Subject Headings (MeSH)
- Diphtheria-Tetanus-Pertussis Vaccine at the US National Library of Medicine Medical Subject Headings (MeSH)
- Diphtheria-Tetanus-acellular Pertussis Vaccines at the US National Library of Medicine Medical Subject Headings (MeSH)
 |

