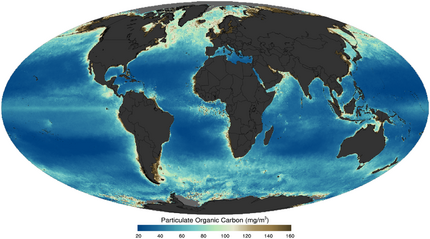
Earth:Particulate organic matter
Particulate organic matter (POM) is a fraction of total organic matter operationally defined as that which does not pass through a filter pore size that typically ranges in size from 0.053 millimeters (53 μm) to 2 millimeters.[3]
Particulate organic carbon (POC) is a closely related term often used interchangeably with POM. POC refers specifically to the mass of carbon in the particulate organic material, while POM refers to the total mass of the particulate organic matter. In addition to carbon, POM includes the mass of the other elements in the organic matter, such as nitrogen, oxygen and hydrogen. In this sense POC is a component of POM and there is typically about twice as much POM as POC.[4] Many statements that can be made about POM apply equally to POC, and much of what is said in this article about POM could equally have been said of POC.
Particulate organic matter is sometimes called suspended organic matter, macroorganic matter, or coarse fraction organic matter. When land samples are isolated by sieving or filtration, this fraction includes partially decomposed detritus and plant material, pollen, and other materials.[5][6] When sieving to determine POM content, consistency is crucial because isolated size fractions will depend on the force of agitation.[7]
POM is readily decomposable, serving many soil functions and providing terrestrial material to water bodies. It is a source of food for both soil organisms and aquatic organisms and provides nutrients for plants. In water bodies, POM can contribute substantially to turbidity, limiting photic depth which can suppress primary productivity. POM also enhances soil structure leading to increased water infiltration, aeration and resistance to erosion.[5][8] Soil management practices, such as tillage and compost/manure application, alter the POM content of soil and water.[5][6]
Overview
Particulate organic carbon (POC) is operationally defined as all combustible, non-carbonate carbon that can be collected on a filter. The oceanographic community has historically used a variety of filters and pore sizes, most commonly 0.7, 0.8, or 1.0 μm glass or quartz fiber filters. The biomass of living zooplankton is intentionally excluded from POC through the use of a pre-filter or specially designed sampling intakes that repel swimming organisms.[9] Sub-micron particles, including most marine prokaryotes, which are 0.2–0.8 μm in diameter, are often not captured but should be considered part of POC rather than dissolved organic carbon (DOC), which is usually operationally defined as < 0.2 μm.[10][9] Typically POC is considered to contain suspended and sinking particles ≥ 0.2 μm in size, which therefore includes biomass from living microbial cells, detrital material including dead cells, fecal pellets, other aggregated material, and terrestrially-derived organic matter. Some studies further divide POC operationally based on its sinking rate or size,[11] with ≥ 51 μm particles sometimes equated to the sinking fraction.[12] Both DOC and POC play major roles in the carbon cycle, but POC is the major pathway by which organic carbon produced by phytoplankton is exported – mainly by gravitational settling – from the surface to the deep ocean and eventually to sediments, and is thus a key component of the biological pump.[13][14][15][16][17][9]
Terrestrial ecosystems
Soil organic matter
Soil organic matter is anything in the soil of biological origin. Carbon is its key component comprising about 58% by weight. Simple assessment of total organic matter is obtained by measuring organic carbon in soil. Living organisms (including roots) contribute about 15% of the total organic matter in soil. These are critical to operation of the soil carbon cycle. What follows refers to the remaining 85% of the soil organic matter - the non-living component.[18]
As shown below, non-living organic matter in soils can be grouped into four distinct categories on the basis of size, behaviour and persistence.[19] These categories are arranged in order of decreasing ability to decompose. Each of them contribute to soil health in different ways.[19][18]
| Soil organic matter |
| ||||||||||||||||||
| (non‑living) |
Dissolved organic matter (DOM): is the organic matter which dissolves in soil water. It comprises the relatively simple organic compounds (e.g. organic acids, sugars and amino acids) which easily decompose. It has a turnover time of less than 12 months. Exudates from plant roots (mucilages and gums) are included here.[18]
Particulate organic matter (POM): is the organic matter that retains evidence of its original cellular structure,[18] and is discussed further in the next section.
Humus: is usually the largest proportion of organic matter in soil, contributing 45 to 75%. Typically it adheres to soil minerals, and plays an important role structuring soil. Humus is the end product of soil organism activity, is chemically complex, and does not have recognisable characteristics of its origin. Humus is of very small unit size and has large surface area in relation to its weight. It holds nutrients, has high water holding capacity and significant cation exchange capacity, buffers pH change and can hold cations. Humus is quite slow to decompose and exists in soil for decades.[18]
Resistant organic matter: has a high carbon content and includes charcoal, charred plant materials, graphite and coal. Turnover times are long and estimated in hundreds of years. It is not biologically active but contributes positively to soil structural properties, including water holding capacity, cation exchange capacity and thermal properties.[18]
Role of POM in soils
Particulate organic matter (POM) includes steadily decomposing plant litter and animal faeces, and the detritus from the activity of microorganisms. Most of it continually undergoes decomposition by microorganisms (when conditions are sufficiently moist) and usually has a turnover time of less than 10 years. Less active parts may take 15 to 100 years to turnover. Where it is still at the soil surface and relatively fresh, particulate organic matter intercepts the energy of raindrops and protects physical soil surfaces from damage. As it is decomposes, particulate organic matter provides much of the energy required by soil organisms as well as providing a steady release of nutrients into the soil environment.[18]
The decomposition of POM provides energy and nutrients. Nutrients not taken up by soil organisms may be available for plant uptake.[6] The amount of nutrients released (mineralized) during decomposition depends on the biological and chemical characteristics of the POM, such as the C:N ratio.[6] In addition to nutrient release, decomposers colonizing POM play a role in improving soil structure.[20] Fungal mycelium entangle soil particles and release sticky, cement-like, polysaccharides into the soil; ultimately forming soil aggregates [20]
Soil POM content is affected by organic inputs and the activity of soil decomposers. The addition of organic materials, such as manure or crop residues, typically results in an increase in POM.[6] Alternatively, repeated tillage or soil disturbance increases the rate of decomposition by exposing soil organisms to oxygen and organic substrates; ultimately, depleting POM. Reduction in POM content is observed when native grasslands are converted to agricultural land.[5] Soil temperature and moisture also affect the rate of POM decomposition.[6] Because POM is a readily available (labile) source of soil nutrients, is a contributor to soil structure, and is highly sensitive to soil management, it is frequently used as an indicator to measure soil quality.[8]
Freshwater ecosystems
In poorly-managed soils, particularly on sloped ground, erosion and transport of soil sediment rich in POM can contaminate water bodies.[8] Because POM provides a source of energy and nutrients, rapid build-up of organic matter in water can result in eutrophication.[20] Suspended organic materials can also serve as a potential vector for the pollution of water with fecal bacteria, toxic metals or organic compounds.
Marine ecosystems


Life and particulate organic matter in the ocean have fundamentally shaped the planet. On the most basic level, particulate organic matter can be defined as both living and non-living matter of biological origin with a size of ≥0.2 μm in diameter, including anything from a small bacterium (0.2 μm in size) to blue whales (20 m in size).[22] Organic matter plays a crucial role in regulating global marine biogeochemical cycles and events, from the Great Oxidation Event in Earth's early history [23] to the sequestration of atmospheric carbon dioxide in the deep ocean.[24] Understanding the distribution, characteristics, dynamics, and changes over time of particulate matter in the ocean is hence fundamental in understanding and predicting the marine ecosystem, from food web dynamics to global biogeochemical cycles.[25][26]
Measuring POM
Optical particle measurements are emerging as an important technique for understanding the ocean carbon cycle, including contributions to estimates of their downward flux, which sequesters carbon dioxide in the deep sea. Optical instruments can be used from ships or installed on autonomous platforms, delivering much greater spatial and temporal coverage of particles in the mesopelagic zone of the ocean than traditional techniques, such as sediment traps. Technologies to image particles have advanced greatly over the last two decades, but the quantitative translation of these immense datasets into biogeochemical properties remains a challenge. In particular, advances are needed to enable the optimal translation of imaged objects into carbon content and sinking velocities. In addition, different devices often measure different optical properties, leading to difficulties in comparing results.[25]
Ocean primary production
Marine primary production can be divided into new production from allochthonous nutrient inputs to the euphotic zone, and regenerated production from nutrient recycling in the surface waters. The total new production in the ocean roughly equates to the sinking flux of particulate organic matter to the deep ocean, about 4 × 109 tons of carbon annually.[27]
Model of sinking oceanic particles
Sinking oceanic particles encompass a wide range of shape, porosity, ballast and other characteristics. The model shown in the diagram at the right attempts to capture some of the predominant features that influence the shape of the sinking flux profile (red line).[21] The sinking of organic particles produced in the upper sunlit layers of the ocean forms an important limb of the oceanic biological pump, which impacts the sequestration of carbon and resupply of nutrients in the mesopelagic ocean. Particles raining out from the upper ocean undergo remineralization by bacteria colonized on their surface and interior, leading to an attenuation in the sinking flux of organic matter with depth. The diagram illustrates a mechanistic model for the depth-dependent, sinking, particulate mass flux constituted by a range of sinking, remineralizing particles.[21]
Marine snow varies in shape, size and character, ranging from individual cells to pellets and aggregates, most of which is rapidly colonized and consumed by heterotrophic bacteria, contributing to the attenuation of the sinking flux with depth.[21]
Sinking velocity
The range of recorded sinking velocities of particles in the oceans spans from negative (particles float toward the surface)[28][29] to several km per day (as with salp fecal pellets)[30] When considering the sinking velocity of an individual particle, a first approximation can be obtained from Stoke's law (originally derived for spherical, non-porous particles and laminar flow) combined with White's approximation,[31] which suggest that sinking velocity increases linearly with excess density (the difference from the water density) and the square of particle diameter (i.e., linearly with the particle area). Building on these expectations, many studies have tried to relate sinking velocity primarily to size, which has been shown to be a useful predictor for particles generated in controlled environments (e.g., roller tanks.[32][33][34] However, strong relationships were only observed when all particles were generated using the same water/plankton community.[35] When particles were made by different plankton communities, size alone was a bad predictor (e.g., Diercks and Asper, 1997) strongly supporting notions that particle densities and shapes vary widely depending on the source material.[35][25]
Packaging and porosity contribute appreciably to determining sinking velocities. On the one hand, adding ballasting materials, such as diatom frustules, to aggregates may lead to an increase in sinking velocities owing to the increase in excess density. On the other hand, the addition of ballasting mineral particles to marine particle populations frequently leads to smaller more densely packed aggregates that sink slower because of their smaller size.[36][37] Mucous-rich particles have been shown to float despite relatively large sizes,[28][38] whereas oil- or plastic-containing aggregates have been shown to sink rapidly despite the presence of substances with an excess density smaller than seawater.[39][40] In natural environments, particles are formed through different mechanisms, by different organisms, and under varying environmental conditions that affect aggregation (e.g., salinity, pH, minerals), ballasting (e.g., dust deposition, sediment load;[35][34] van der Jagt et al., 2018) and sinking behaviour (e.g., viscosity;[41]). A universal conversion of size-to-sinking velocity is hence impracticable.[42][25]
Role in the lower aquatic food web
Along with dissolved organic matter, POM drives the lower aquatic food web by providing energy in the form of carbohydrates, sugars, and other polymers that can be degraded. POM in water bodies is derived from terrestrial inputs (e.g. soil organic matter, leaf litterfall), submerged or floating aquatic vegetation, or autochthonous production of algae (living or detrital). Each source of POM has its own chemical composition that affects its lability, or accessibility to the food web. Algal-derived POM is thought to be most labile, but there is growing evidence that terrestrially-derived POM can supplement the diets of micro-organisms such as zooplankton when primary productivity is limited.[43][44]
The biological carbon pump
The dynamics of the particulate organic carbon (POC) pool in the ocean are central to the marine carbon cycle. POC is the link between surface primary production, the deep ocean, and sediments. The rate at which POC is degraded in the dark ocean can impact atmospheric CO2 concentration. Therefore, a central focus of marine organic geochemistry studies is to improve the understanding of POC distribution, composition, and cycling. The last few decades have seen improvements in analytical techniques that have greatly expanded what can be measured, both in terms of organic compound structural diversity and isotopic composition, and complementary molecular omics studies.[9]

As illustrated in the diagram, phytoplankton fix carbon dioxide in the euphotic zone using solar energy and produce POC. POC formed in the euphotic zone is processed by marine microorganisms (microbes), zooplankton and their consumers into organic aggregates (marine snow), which is then exported to the mesopelagic (200–1000 m depth) and bathypelagic zones by sinking and vertical migration by zooplankton and fish.[46][47][48]
The biological carbon pump describes the collection of biogeochemical processes associated with the production, sinking, and remineralization of organic carbon in the ocean.[49][50] In brief, photosynthesis by microorganisms in the upper tens of meters of the water column fix inorganic carbon (any of the chemical species of dissolved carbon dioxide) into biomass. When this biomass sinks to the deep ocean, a portion of it fuels the metabolism of the organisms living there, including deep-sea fish and benthic organisms.[48] Zooplankton play a critical role in shaping particle flux through ingestion and fragmentation of particles,[51][52][53][54][55][56] production of fast-sinking fecal material[48][30] and active vertical migration.[57][58][59][25]
Besides the importance of "exported" organic carbon as a food source for deep ocean organisms, the biological carbon pump provides a valuable ecosystem function: Exported organic carbon transports an estimated 5–20 Gt C each year to the deep ocean,[60] where some of it (~0.2–0.5 Gt C)[61] is sequestered for several millennia. The biological carbon pump is hence of similar magnitude to current carbon emissions from fossil fuels (~10 Gt C year−1). Any changes in its magnitude caused by a warming world may have direct implications for both deep-sea organisms and atmospheric carbon dioxide concentrations.[62][47][25]
The magnitude and efficiency (amount of carbon sequestered relative to primary production) of the biological carbon pump, hence ocean carbon storage, is partly determined by the amount of organic matter exported and the rate at which it is remineralized (i.e., the rate with which sinking organic matter is reworked and respired in the mesopelagic zone region.[62][63][64] Especially particle size and composition are important parameters determining how fast a particle sinks,[65][63] how much material it contains,[66] and which organisms can find and utilize it.[67][68][69][25]
Sinking particles can be phytoplankton, zooplankton, detritus, fecal pellets, or a mix of these.[70][71][48] They range in size from a few micrometers to several centimeters, with particles of a diameter of >0.5 mm being referred to as marine snow.[72] In general, particles in a fluid are thought to sink once their densities are higher than the ambient fluid, i.e., when excess densities are larger than zero. Larger individual phytoplankton cells can thus contribute to sedimentary fluxes. For example, large diatom cells and diatom chains with a diameter of >5 μm have been shown to sink at rates up to several 10 s meters per day, though this is only possible owing to the heavy ballast of a silica frustule.[73][74] Both size and density affect particle sinking velocity; for example, for sinking velocities that follow Stokes' Law, doubling the size of the particle increases the sinking speed by a factor of 4.[75][73] However, the highly porous nature of many marine particles means that they do not obey Stokes' Law because small changes in particle density (i.e., compactness) can have a large impact on their sinking velocities.[63] Large sinking particles are typically of two types: (1) aggregates formed from a number of primary particles, including phytoplankton, bacteria, fecal pellets, live protozoa and zooplankton and debris, and (2) zooplankton fecal pellets, which can dominate particle flux events and sink at velocities exceeding 1,000 m d−1.[48][25]
Knowing the size, abundance, structure and composition (e.g. carbon content) of settling particles is important as these characteristics impose fundamental constraints on the biogeochemical cycling of carbon. For example, changes in climate are expected to facilitate a shift in species composition in a manner that alters the elemental composition of particulate matter, cell size and the trajectory of carbon through the food web, influencing the proportion of biomass exported to depth.[76] As such, any climate-induced change in the structure or function of phytoplankton communities is likely to alter the efficiency of the biological carbon pump, with feedbacks on the rate of climate change.[77][78][25]
Bioluminescent shunt hypothesis
The consumption of the bioluminescent POC by fish can lead to the emission of bioluminescent fecal pellets (repackaging), which can also be produced with non-bioluminescent POC if the fish gut is already charged with bioluminescent bacteria.[80]
In the diagram on the right, the sinking POC is moving downward followed by a chemical plume.[81] The plain white arrows represent the carbon flow. Panel (a) represents the classical view of a non-bioluminescent particle. The length of the plume is identified by the scale on the side.[82] Panel (b) represents the case of a glowing particle in the bioluminescence shunt hypothesis. Bioluminescent bacteria are represented aggregated onto the particle. Their light emission is shown as a bluish cloud around it. Blue dotted arrows represent the visual detection and the movement toward the particle of the consumer organisms. Increasing the visual detection allows a better detection by upper trophic levels, potentially leading to the fragmentation of sinking POC into suspended POC due to sloppy feeding.[80]
See also
- Microbial loop
- Particulate matter
- Total organic carbon
References
- ↑ Monroy, P., Hernández-García, E., Rossi, V. and López, C. (2017) "Modeling the dynamical sinking of biogenic particles in oceanic flow". Nonlinear Processes in Geophysics, 24(2): 293–305. doi:10.5194/npg-24-293-2017. 50px Material was copied from this source, which is available under a Creative Commons Attribution 3.0 International License.
- ↑ Simon, M., Grossart, H., Schweitzer, B. and Ploug, H. (2002) "Microbial ecology of organic aggregates in aquatic ecosystems". Aquatic microbial ecology, 28: 175–211. doi:10.3354/ame028175.
- ↑ Cambardella, C. A.; Elliott, E. T. (1991). "Particulate soil organic-matter changes across a grassland cultivation sequence.". Soil Science Society of America Journal 56 (3): 777–783. doi:10.2136/sssaj1992.03615995005600030017x.
- ↑ Moody, C.S. and Worrall, F. (2017) "Modeling rates of DOC degradation using DOM composition and hydroclimatic variables". Journal of Geophysical Research: Biogeosciences, 122(5): 1175–1191. doi:10.1002/2016JG003493.
- ↑ 5.0 5.1 5.2 5.3 Brady, N. C.; Weil, R. R. (2007). The nature and properties of soils (11th ed.). Upper Saddle River, NJ: Prentice-Hall Inc..
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Gregorich, E. G.; Beare, M. H.; McKim, U. F.; Skjemstad, J. O. (2006). "Chemical and biological characteristics of physically uncomplexed organic matter". Soil Science Society of America Journal 70 (3): 975–985. doi:10.2136/sssaj2005.0116. Bibcode: 2006SSASJ..70..975G.
- ↑ Carter, M. R. (1993). Soil Sampling and Methods of Analysis. CRC Press.
- ↑ 8.0 8.1 8.2 "Particulate Organic Matter". NRCS. http://soilquality.org/indicators/pom.html.
- ↑ 9.0 9.1 9.2 9.3 9.4 Kharbush, J.J., Close, H.G., Van Mooy, B.A., Arnosti, C., Smittenberg, R.H., Le Moigne, F.A., Mollenhauer, G., Scholz-Böttcher, B., Obreht, I., Koch, B.P. and Becker, K. (2020) "Particulate Organic Carbon Deconstructed: Molecular and Chemical Composition of Particulate Organic Carbon in the Ocean". Frontiers in Marine Science, 7: 518. doi:10.3389/fmars.2020.00518. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Wagner, Sasha; Schubotz, Florence; Kaiser, Karl; Hallmann, Christian; Waska, Hannelore; Rossel, Pamela E.; Hansman, Roberta; Elvert, Marcus et al. (2020). "Soothsaying DOM: A Current Perspective on the Future of Oceanic Dissolved Organic Carbon". Frontiers in Marine Science 7. doi:10.3389/fmars.2020.00341.
- ↑ Riley, J. S.; Sanders, R.; Marsay, C.; Le Moigne, F. A. C.; Achterberg, E. P.; Poulton, A. J. (2012). "The relative contribution of fast and slow sinking particles to ocean carbon export". Global Biogeochemical Cycles 26 (1): n/a. doi:10.1029/2011GB004085. Bibcode: 2012GBioC..26.1026R.
- ↑ Lam, Phoebe J.; Doney, Scott C.; Bishop, James K. B. (2011). "The dynamic ocean biological pump: Insights from a global compilation of particulate organic carbon, CaCO3, and opal concentration profiles from the mesopelagic". Global Biogeochemical Cycles 25 (3): n/a. doi:10.1029/2010GB003868. Bibcode: 2011GBioC..25.3009L.
- ↑ Eppley, Richard W.; Peterson, Bruce J. (1979). "Particulate organic matter flux and planktonic new production in the deep ocean". Nature 282 (5740): 677–680. doi:10.1038/282677a0. Bibcode: 1979Natur.282..677E.
- ↑ Volk, Tyler; Hoffert, Martin I. (2013). "Ocean Carbon Pumps: Analysis of Relative Strengths and Efficiencies in Ocean-Driven Atmospheric CO2 Changes". The Carbon Cycle and Atmospheric CO2 : Natural Variations Archean to Present. Geophysical Monograph Series. pp. 99–110. doi:10.1029/gm032p0099. ISBN 9781118664322.
- ↑ Boyd, P.W.; Trull, T.W. (2007). "Understanding the export of biogenic particles in oceanic waters: Is there consensus?". Progress in Oceanography 72 (4): 276–312. doi:10.1016/j.pocean.2006.10.007. Bibcode: 2007PrOce..72..276B. https://figshare.com/articles/journal_contribution/22859495.
- ↑ Cavan, E. L.; Le Moigne, F. A. C.; Poulton, A. J.; Tarling, G. A.; Ward, P.; Daniels, C. J.; Fragoso, G. M.; Sanders, R. J. (2015). "Attenuation of particulate organic carbon flux in the Scotia Sea, Southern Ocean, is controlled by zooplankton fecal pellets". Geophysical Research Letters 42 (3): 821–830. doi:10.1002/2014GL062744. Bibcode: 2015GeoRL..42..821C.
- ↑ Le Moigne, Frédéric A. C. (2019). "Pathways of Organic Carbon Downward Transport by the Oceanic Biological Carbon Pump". Frontiers in Marine Science 6. doi:10.3389/fmars.2019.00634.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 Soil: Forms and Function Victorian Resources Online. Updated 23 March 2020.
 Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 19.0 19.1 Baldock JA and Skjemstad JO (1999) "Soil organic carbon/soil organic matter in soil". In KI Peverill, LA Sparrow and DJ Reuter (Eds.) Soil analysis: an interpretation manual, pages 159–170, Commonwealth Scientific and Industrial Research Organisation, Melbourne. ISBN:9780643063761
- ↑ 20.0 20.1 20.2 Six, J.; Bossuyt, H.; Degryze, S; Denef, K (2004). "A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics". Soil and Tillage Research 79 (1): 7–31. doi:10.1016/j.still.2004.03.008.
- ↑ 21.0 21.1 21.2 21.3 Omand, M.M., Govindarajan, R., He, J. and Mahadevan, A. (2020) "Sinking flux of particulate organic matter in the oceans: Sensitivity to particle characteristics". Scientific Reports, 10(1): 1–16. doi:10.1038/s41598-020-60424-5. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Blanchard, J.L., Heneghan, R.F., Everett, J.D., Trebilco, R. and Richardson, A.J. (2017) "From bacteria to whales: using functional size spectra to model marine ecosystems. Trends in ecology & evolution, 32(3), pp.174-186. doi:10.1016/j.tree.2016.12.003.
- ↑ Holland, H.D. (2006) "The oxygenation of the atmosphere and oceans". Philosophical Transactions of the Royal Society B: Biological Sciences, 361(1470): 903–915. doi:10.1098/rstb.2006.1838.
- ↑ Heinze, C., Meyer, S., Goris, N., Anderson, L., Steinfeldt, R., Chang, N., Quéré, C.L. and Bakker, D.C. (2015) "The ocean carbon sink–impacts, vulnerabilities and challenges". Earth System Dynamics, 6(1): 327–358. doi:10.5194/esd-6-327-2015.
- ↑ 25.0 25.1 25.2 25.3 25.4 25.5 25.6 25.7 25.8 Giering, S.L., Cavan, E.L., Basedow, S.L., Briggs, N., Burd, A.B., Darroch, L.J., Guidi, L., Irrison, J.O., Iversen, M.H., Kiko, R. and Lindsay, D.J. (2020) "Sinking organic particles in the ocean—flux estimates from in situ optical devices". doi:10.3389/fmars.2019.00834.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Conte, Maureen H; Ralph, Nate; Ross, Edith H (2001). "Seasonal and interannual variability in deep ocean particle fluxes at the Oceanic Flux Program (OFP)/Bermuda Atlantic Time Series (BATS) site in the western Sargasso Sea near Bermuda" (in en). Deep Sea Research Part II: Topical Studies in Oceanography 48 (8–9): 1471–1505. doi:10.1016/S0967-0645(00)00150-8. Bibcode: 2001DSRII..48.1471C. https://linkinghub.elsevier.com/retrieve/pii/S0967064500001508.
- ↑ Eppley, R.W. and Peterson, B.J. (1979) "Particulate organic matter flux and planktonic new production in the deep ocean". Nature, 282(5740): 677–680. doi:10.1038/282677a0.
- ↑ 28.0 28.1 Azetsu-Scott, Kumiko; Passow, Uta (2004). "Ascending marine particles: Significance of transparent exopolymer particles (TEP) in the upper ocean". Limnology and Oceanography 49 (3): 741–748. doi:10.4319/lo.2004.49.3.0741. Bibcode: 2004LimOc..49..741A. https://epic.awi.de/id/eprint/10032/1/Aze2004a.pdf.
- ↑ Acuña, JL; López-Alvarez, M.; Nogueira, E.; González-Taboada, F. (2010). "Diatom flotation at the onset of the spring phytoplankton bloom: An in situ experiment". Marine Ecology Progress Series 400: 115–125. doi:10.3354/meps08405. Bibcode: 2010MEPS..400..115A.
- ↑ 30.0 30.1 Iversen, M.H., Pakhomov, E.A., Hunt, B.P., Van der Jagt, H., Wolf-Gladrow, D. and Klaas, C. (2017) "Sinkers or floaters? Contribution from salp pellets to the export flux during a large bloom event in the Southern Ocean". Deep Sea Research Part II: Topical Studies in Oceanography, 138: 116–125. doi:10.1016/j.dsr2.2016.12.004.
- ↑ White, Frank M. (2006). Viscous Fluid Flow. McGraw-Hill. ISBN 9780071244930. https://books.google.com/books?id=fl6wPwAACAAJ&q=%22Viscous+Fluid+Flow%22+Mechanical+Engineering.
- ↑ Gärdes, Astrid; Iversen, Morten H.; Grossart, Hans-Peter; Passow, Uta; Ullrich, Matthias S. (2011). "Diatom-associated bacteria are required for aggregation of Thalassiosira weissflogii". The ISME Journal 5 (3): 436–445. doi:10.1038/ismej.2010.145. PMID 20827289.
- ↑ Iversen, M. H.; Ploug, H. (2013). "Temperature effects on carbon-specific respiration rate and sinking velocity of diatom aggregates – potential implications for deep ocean export processes". Biogeosciences 10 (6): 4073–4085. doi:10.5194/bg-10-4073-2013. Bibcode: 2013BGeo...10.4073I.
- ↑ 34.0 34.1 Iversen, Morten H.; Robert, Maya L. (2015). "Ballasting effects of smectite on aggregate formation and export from a natural plankton community". Marine Chemistry 175: 18–27. doi:10.1016/j.marchem.2015.04.009. Bibcode: 2015MarCh.175...18I.
- ↑ 35.0 35.1 35.2 Iversen, Morten Hvitfeldt; Nowald, Nicolas; Ploug, Helle; Jackson, George A.; Fischer, Gerhard (2010). "High resolution profiles of vertical particulate organic matter export off Cape Blanc, Mauritania: Degradation processes and ballasting effects". Deep Sea Research Part I: Oceanographic Research Papers 57 (6): 771–784. doi:10.1016/j.dsr.2010.03.007. Bibcode: 2010DSRI...57..771I.
- ↑ Hamm, Christian E. (2002). "Interactive aggregation and sedimentation of diatoms and clay‐sized lithogenic material". Limnology and Oceanography 47 (6): 1790–1795. doi:10.4319/lo.2002.47.6.1790. Bibcode: 2002LimOc..47.1790H.
- ↑ Passow, Uta; de la Rocha, Christina L.; Fairfield, Caitlin; Schmidt, Katrin (2014). "Aggregation as a function of and mineral particles". Limnology and Oceanography 59 (2): 532–547. doi:10.4319/lo.2014.59.2.0532. Bibcode: 2014LimOc..59..532P.
- ↑ Bochdansky, Alexander B.; Clouse, Melissa A.; Herndl, Gerhard J. (2016). "Dragon kings of the deep sea: Marine particles deviate markedly from the common number-size spectrum". Scientific Reports 6: 22633. doi:10.1038/srep22633. PMID 26940454. Bibcode: 2016NatSR...622633B.
- ↑ Long, Marc; Moriceau, Brivaëla; Gallinari, Morgane; Lambert, Christophe; Huvet, Arnaud; Raffray, Jean; Soudant, Philippe (2015). "Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates". Marine Chemistry 175: 39–46. doi:10.1016/j.marchem.2015.04.003. Bibcode: 2015MarCh.175...39L. https://archimer.ifremer.fr/doc/00259/37048/.
- ↑ Passow, U.; Sweet, J.; Francis, S.; Xu, C.; Dissanayake, AL; Lin, YY; Santschi, PH; Quigg, A. (2019). "Incorporation of oil into diatom aggregates". Marine Ecology Progress Series 612: 65–86. doi:10.3354/meps12881. Bibcode: 2019MEPS..612...65P.
- ↑ Taucher, J.; Bach, L. T.; Riebesell, U.; Oschlies, A. (2014). "The viscosity effect on marine particle flux: A climate relevant feedback mechanism". Global Biogeochemical Cycles 28 (4): 415–422. doi:10.1002/2013GB004728. Bibcode: 2014GBioC..28..415T. http://oceanrep.geomar.de/24797/1/gbc20151.pdf.
- ↑ Jouandet, Marie-Paule; Trull, Thomas W.; Guidi, Lionel; Picheral, Marc; Ebersbach, Friederike; Stemmann, Lars; Blain, Stéphane (2011). "Optical imaging of mesopelagic particles indicates deep carbon flux beneath a natural iron-fertilized bloom in the Southern Ocean". Limnology and Oceanography 56 (3): 1130–1140. doi:10.4319/lo.2011.56.3.1130. Bibcode: 2011LimOc..56.1130J.
- ↑ Weidel, Brian; Solomon, Christopher T.; Pace, Michael L.; Kitchell, Jim; Carpenter, Stephen R.; Cole, Jonathan J. (2011-02-01). "Strong evidence for terrestrial support of zooplankton in small lakes based on stable isotopes of carbon, nitrogen, and hydrogen" (in en). Proceedings of the National Academy of Sciences 108 (5): 1975–1980. doi:10.1073/pnas.1012807108. ISSN 0027-8424. PMID 21245299. Bibcode: 2011PNAS..108.1975C.
- ↑ Kankaala, Paula; Strandberg, Ursula; Kimmo K. Kahilainen; Aalto, Sanni L.; Galloway, Aaron W. E.; Taipale, Sami J. (2016-08-11). "Terrestrial carbohydrates support freshwater zooplankton during phytoplankton deficiency" (in en). Scientific Reports 6: 30897. doi:10.1038/srep30897. ISSN 2045-2322. PMID 27510848. Bibcode: 2016NatSR...630897T.
- ↑ Henley, Sian F.; Cavan, Emma L.; Fawcett, Sarah E.; Kerr, Rodrigo; Monteiro, Thiago; Sherrell, Robert M.; Bowie, Andrew R.; Boyd, Philip W. et al. (2020). "Changing Biogeochemistry of the Southern Ocean and Its Ecosystem Implications". Frontiers in Marine Science 7. doi:10.3389/fmars.2020.00581. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Basu, S. and Mackey, K.R. (2018) "Phytoplankton as key mediators of the biological carbon pump: Their responses to a changing climate". Sustainability, 10(3): 869. doi:10.3390/su10030869.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 47.0 47.1 Passow, U. and Carlson, C.A. (2012) "The biological pump in a high CO2 world". Marine Ecology Progress Series, 470: 249–271. doi:10.3354/meps09985.
- ↑ 48.0 48.1 48.2 48.3 48.4 Turner, Jefferson T. (2015). "Zooplankton fecal pellets, marine snow, phytodetritus and the ocean's biological pump". Progress in Oceanography 130: 205–248. doi:10.1016/j.pocean.2014.08.005. Bibcode: 2015PrOce.130..205T.
- ↑ Volk, T. and Hoffert, M.I. (1985) "Ocean carbon pumps: Analysis of relative strengths and efficiencies in ocean‐driven atmospheric CO2 changes. In: The carbon cycle and atmospheric CO2: natural variations Archean to present, pages 99–110, University of California. ISBN:9780875900605.
- ↑ Giering, S.L. and Humphreys, M.P. (2018) "Biological Pump". In: Encyclopedia of Geochemistry, W. White (Ed.) Cham: Springer, pages 1–6. doi:10.1007/978-3-319-39193-9_154-1.
- ↑ Waite, A.M., Safi, K.A., Hall, J.A. and Nodder, S.D. (2000) "Mass sedimentation of picoplankton embedded in organic aggregates". Limnology and Oceanography, 45(1): 87–97. doi:10.4319/lo.2000.45.1.0087.
- ↑ Iversen, M.H. and Poulsen, L.K. (2007) "Coprorhexy, coprophagy, and coprochaly in the copepods Calanus helgolandicus, Pseudocalanus elongatus, and Oithona similis". Marine Ecology Progress Series, 350: 79–89. doi:10.3354/meps07095.
- ↑ Poulsen, L.K. and Iversen, M.H. (2008) "Degradation of copepod fecal pellets: key role of protozooplankton". Marine Ecology Progress Series, 367: 1–13. doi:10.3354/meps07611.
- ↑ Iversen, M.H., Nowald, N., Ploug, H., Jackson, G.A. and Fischer, G. (2010) "High resolution profiles of vertical particulate organic matter export off Cape Blanc, Mauritania: Degradation processes and ballasting effects". Deep Sea Research Part I: Oceanographic Research Papers, 57(6): 771–784. doi:10.1016/j.dsr.2010.03.007.
- ↑ Giering, S.L., Sanders, R., Lampitt, R.S., Anderson, T.R., Tamburini, C., Boutrif, M., Zubkov, M.V., Marsay, C.M., Henson, S.A., Saw, K. and Cook, K. (2014) "Reconciliation of the carbon budget in the ocean’s twilight zone". Nature, 507(7493): 480–483. doi:10.1038/nature13123.
- ↑ Svensen, C., Morata, N. and Reigstad, M. (2014) "Increased degradation of copepod faecal pellets by co-acting dinoflagellates and Centropages hamatus". Marine Ecology Progress Series, 516: 61–70. doi:10.3354/meps10976.
- ↑ Steinberg, D.K., Carlson, C.A., Bates, N.R., Goldthwait, S.A., Madin, L.P. and Michaels, A.F. (2000) "Zooplankton vertical migration and the active transport of dissolved organic and inorganic carbon in the Sargasso Sea". Deep Sea Research Part I: Oceanographic Research Papers, 47(1): 137–158. doi:10.1016/S0967-0637(99)00052-7.
- ↑ Jónasdóttir, S.H., Visser, A.W., Richardson, K. and Heath, M.R. (2015) "Seasonal copepod lipid pump promotes carbon sequestration in the deep North Atlantic". Proceedings of the National Academy of Sciences, 112(39): 12122–12126. doi:10.1073/pnas.1512110112.
- ↑ Kiko, R., Biastoch, A., Brandt, P., Cravatte, S., Hauss, H., Hummels, R., Kriest, I., Marin, F., McDonnell, A.M., Oschlies, A. and Picheral, M. (2017) "Biological and physical influences on marine snowfall at the equator". Nature Geoscience, 10(11): 852–858. doi:10.1038/ngeo3042.
- ↑ Henson, S.A., Sanders, R., Madsen, E., Morris, P.J., Le Moigne, F. and Quartly, G.D. (2011) "A reduced estimate of the strength of the ocean's biological carbon pump". Geophysical Research Letters, 38(4). doi:10.1029/2011GL046735.
- ↑ Guidi, L., Legendre, L., Reygondeau, G., Uitz, J., Stemmann, L. and Henson, S.A. (2015) "A new look at ocean carbon remineralization for estimating deepwater sequestration". Global Biogeochemical Cycles, 29(7): 1044–1059. doi:10.1002/2014GB005063.
- ↑ 62.0 62.1 Kwon, E.Y., Primeau, F. and Sarmiento, J.L. (2009) "The impact of remineralization depth on the air–sea carbon balance". Nature Geoscience, 2(9): 630–635. doi:10.1038/ngeo612.
- ↑ 63.0 63.1 63.2 Iversen, M. and Ploug, H. (2010) "Ballast minerals and the sinking carbon flux in the ocean: carbon-specific respiration rates and sinking velocity of marine snow aggregates". Biogeosciences, 7: 2613–2624. doi:10.5194/bg-7-2613-2010.
- ↑ Reygondeau, G., Guidi, L., Beaugrand, G., Henson, S.A., Koubbi, P., MacKenzie, B.R., Sutton, T.T., Fioroni, M. and Maury, O. (2018) "Global biogeochemical provinces of the mesopelagic zone". Journal of Biogeography, 45(2): 500–514. doi:10.1111/jbi.13149.
- ↑ Ploug, H., Iversen, M.H., Koski, M. and Buitenhuis, E.T. (2008) "Production, oxygen respiration rates, and sinking velocity of copepod fecal pellets: direct measurements of ballasting by opal and calcite". Limnology and Oceanography, 53(2): 469–476. doi:10.4319/lo.2008.53.2.0469.
- ↑ Ploug, H., Iversen, M.H. and Fischer, G. (2008) "Ballast, sinking velocity, and apparent diffusivity within marine snow and zooplankton fecal pellets: Implications for substrate turnover by attached bacteria". Limnology and Oceanography, 53(5): 1878–1886. doi:10.4319/lo.2008.53.5.1878.
- ↑ Kiørboe, T., Saiz, E. and Visser, A. (1999) "Hydrodynamic signal perception in the copepod Acartia tonsa". Marine Ecology Progress Series, 179: 97–111. doi:10.3354/meps179097.
- ↑ Visser, A.W. (2001) "Hydromechanical signals in the plankton". Marine Ecology Progress Series, 222: 1–24. doi:10.3354/meps222001.
- ↑ Visser, A.W. and Jackson, G.A. (2004) "Characteristics of the chemical plume behind a sinking particle in a turbulent water column". Marine Ecology Progress Series, 283: 55–71. doi:10.3354/meps283055.
- ↑ Simon, M.; Grossart, HP; Schweitzer, B.; Ploug, H. (2002). "Microbial ecology of organic aggregates in aquatic ecosystems". Aquatic Microbial Ecology 28: 175–211. doi:10.3354/ame028175.
- ↑ Turner, JT (2002). "Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms". Aquatic Microbial Ecology 27: 57–102. doi:10.3354/ame027057.
- ↑ Alldredge, Alice L.; Silver, Mary W. (1988). "Characteristics, dynamics and significance of marine snow". Progress in Oceanography 20 (1): 41–82. doi:10.1016/0079-6611(88)90053-5. Bibcode: 1988PrOce..20...41A.
- ↑ 73.0 73.1 Waite, A.; Fisher, A.; Thompson, PA; Harrison, PJ (1997). "Sinking rate versus cell volume relationships illuminate sinking rate control mechanisms in marine diatoms". Marine Ecology Progress Series 157: 97–108. doi:10.3354/meps157097. Bibcode: 1997MEPS..157...97W.
- ↑ Miklasz, Kevin A.; Denny, Mark W. (2010). "Diatom sinkings speeds: Improved predictions and insight from a modified Stokes' law". Limnology and Oceanography 55 (6): 2513–2525. doi:10.4319/lo.2010.55.6.2513. Bibcode: 2010LimOc..55.2513M.
- ↑ Moore, J. Keith; Villareal, Tracy A. (1996). "Size-ascent rate relationships in positively buoyant marine diatoms". Limnology and Oceanography 41 (7): 1514–1520. doi:10.4319/lo.1996.41.7.1514. Bibcode: 1996LimOc..41.1514M.
- ↑ Finkel, Z. V.; Beardall, J.; Flynn, K. J.; Quigg, A.; Rees, T. A. V.; Raven, J. A. (2010). "Phytoplankton in a changing world: Cell size and elemental stoichiometry". Journal of Plankton Research 32: 119–137. doi:10.1093/plankt/fbp098.
- ↑ Matear, Richard J.; Hirst, Anthony C. (1999). "Climate change feedback on the future oceanic CO2 uptake". Tellus B: Chemical and Physical Meteorology 51 (3): 722–733. doi:10.3402/tellusb.v51i3.16472. Bibcode: 1999TellB..51..722M.
- ↑ Le Quere, C.; Rodenbeck, C.; Buitenhuis, E. T.; Conway, T. J.; Langenfelds, R.; Gomez, A.; Labuschagne, C.; Ramonet, M. et al. (2007). "Saturation of the Southern Ocean CO2 Sink Due to Recent Climate Change". Science 316 (5832): 1735–1738. doi:10.1126/science.1136188. PMID 17510327. Bibcode: 2007Sci...316.1735L.
- ↑ Azam, Farooq; Long, Richard A. (2001). "Sea snow microcosms". Nature 414 (6863): 495–498. doi:10.1038/35107174. PMID 11734832.
- ↑ 80.0 80.1 80.2 Tanet, Lisa; Martini, Séverine; Casalot, Laurie; Tamburini, Christian (2020). "Reviews and syntheses: Bacterial bioluminescence – ecology and impact in the biological carbon pump". Biogeosciences 17 (14): 3757–3778. doi:10.5194/bg-17-3757-2020. Bibcode: 2020BGeo...17.3757T.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Kiørboe, Thomas (2011). "How zooplankton feed: Mechanisms, traits and trade-offs". Biological Reviews 86 (2): 311–339. doi:10.1111/j.1469-185X.2010.00148.x. PMID 20682007.
- ↑ Kiørboe, Thomas; Jackson, George A. (2001). "Marine snow, organic solute plumes, and optimal chemosensory behavior of bacteria". Limnology and Oceanography 46 (6): 1309–1318. doi:10.4319/lo.2001.46.6.1309. Bibcode: 2001LimOc..46.1309K.
 |