Earth:Marl
Marl is an earthy material rich in carbonate minerals, clays, and silt. When hardened into rock, this becomes marlstone. It is formed in marine or freshwater environments, often through the activities of algae.
Marl makes up the lower part of the cliffs of Dover, and the Channel Tunnel follows these marl layers between France and the United Kingdom. Marl is also a common sediment in post-glacial lakes, such as the marl ponds of the northeastern United States.
Marl has been used as a soil conditioner and neutralizing agent for acid soil and in the manufacture of cement.
Description
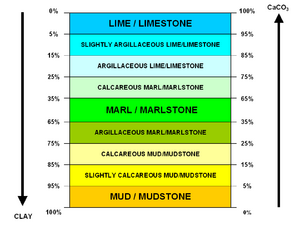
Marl or marlstone is a carbonate-rich mud or mudstone which contains variable amounts of clays and silt. The term was originally loosely applied to a variety of materials, most of which occur as loose, earthy deposits consisting chiefly of an intimate mixture of clay and calcium carbonate,[1] formed under freshwater conditions. These typically contain 35–65% clay and 65–35% carbonate.[2][3] The term is today often used to describe indurated marine deposits and lacustrine (lake) sediments which more accurately should be named 'marlstone'.[4]
Marlstone is an indurated (resists crumbling or powdering) rock of about the same composition as marl. This is more correctly described as an earthy or impure argillaceous limestone. It has a blocky subconchoidal fracture, and is less fissile than shale.[4] The dominant carbonate mineral in most marls is calcite, but other carbonate minerals such as aragonite or dolomite may be present.[5]
Glauconitic marl is marl containing pellets of glauconite, a clay mineral that gives the marl a green color.[6] Glauconite is characteristic of sediments deposited in marine conditions.[7]
Occurrences
The lower stratigraphic units of the chalk cliffs of Dover consist of a sequence of glauconitic marls followed by rhythmically banded limestone and marl layers.[8] Such alternating cycles of chalk and marl are common in Cretaceous beds of northwestern Europe.[9] The Channel Tunnel follows these marl layers between France and the United Kingdom.[10] Upper Cretaceous cyclic sequences in Germany and marl–opal-rich Tortonian-Messinian strata in the Sorbas Basin related to multiple sea drawdown have been correlated with Milankovitch orbital forcing.[11]
Marl as lacustrine sediment is common in post-glacial lake-bed sediments.[12][13][14] Chara, a macroalga also known as stonewort, thrives in shallow lakes with high pH and alkalinity, where its stems and fruiting bodies become calcified. After the alga dies, the calcified stems and fruiting bodies break down into fine carbonate particles that mingle with silt and clay to produce marl.[15] Marl ponds of the northeastern United States are often kettle ponds in areas of limestone bedrock that become poor in nutrients (oligotrophic) due to precipitation of essential phosphate. Normal pond life is unable to survive, and skeletons of freshwater molluscs such as Sphaerium and Planorbis accumulate as part of the bottom marl.[13]
In Hungary, Buda Marl is found that was formed in the Upper Eocene era. It lies between layers of rock and soil and may be defined it as both "weak rock and strong soil."[16]
Economic geology
Marl has been used as a soil conditioner and neutralizing agent for acid soil[13][17] and in the manufacture of Portland cement.[18] Because some marls have a very low permeability, they have been exploited for construction of the Channel Tunnel between England and France and are being investigated for the storage of nuclear waste.
Historical use in agriculture
Marl is one of the oldest soil amendments used in agriculture. In addition to increasing available calcium, marl is valuable for improving soil structure and decreasing soil acidity[19] and thereby making other nutrients more available.[20] It was used sporadically in Britain beginning in prehistoric times[21] and its use was mentioned by Pliny the Elder in the 1st century.[22] Its more widespread use from the 16th century on contributed to the early modern agricultural revolution.[21] However, the lack of a high-energy economy hindered its large-scale use until the Industrial Revolution.[20]
Marl was used extensively in Britain, particularly in Lancashire, during the 18th century. The marl was normally extracted close to its point of use, so that almost every field had a marl pit, but some marl was transported greater distances by railroad. However, marl was gradually replaced by lime and imported mineral fertilizers early in the 19th century.[23] A similar historical pattern was seen in Scotland.[21]
Marl was one a few soil amendments available in limited quantities in the southern United States, where soils were generally poor in nutrients, prior to about 1840.[24] By the late 19th century, marl was being mined on an industrial scale in New Jersey[25] and was increasingly being used on a more scientific basis, with marl being classified by grade[26][27] and the state geological survey publishing detailed chemical analyses.[28]
Modern agricultural and aquacultural uses
Marl continues to be used for agriculture into the 21st century, though less frequently.[29] The rate of application must be adjusted for the reduced content of calcium carbonate versus straight lime, expressed as the calcium carbonate equivalent. Because the carbonate in marl is predominantly calcium carbonate, magnesium deficiency may be seen in crops treated with marl if they are not also supplemented with magnesium.[17]
Marl has been used in Pamlico Sound to provide a suitable artificial substrate for oysters in a reef-like environment.[29]
Portland cement
Marl has been used in the manufacture of Portland cement.[18] It is abundant and yields better physical and mechanical properties than metakaolin as a supplementary cementitious material[30] and can be calcined at a considerably lower temperature.[31][32]
Civil engineering
The Channel Tunnel was constructed in the West Melbury Marly Chalk, a geological formation containing marl beds. This formation was chosen because of its very low permeability, absence of chert, and lack of fissures found in overlying formations. The underlying Glauconitic Marl is easily recognizable in core samples and helped establish the right level for excavating the tunnel.[33]
Marl soil has poor engineering properties, particularly when alternately wetted and dried.[34] The soils can be stabilized by adding pozzolan (volcanic ash) to the soil.[35]
Nuclear waste storage
Some marl beds have a very low permeability and are under consideration for use in the storage of nuclear waste. One such proposed storage site is the Wellenberg in central Switzerland.[36]
Marl lakes
A marl lake is a lake whose bottom sediments include large deposits of marl.[18] They are most often found in areas of recent glaciation[37] and are characterized by alkaline water, rich in dissolved calcium carbonate, from which carbonate minerals are deposited.[38]
Marl lakes have frequently been dredged or mined for marl, often used for manufacturing Portland cement.[18] However, they are regarded as ecologically important,[39] and are vulnerable to damage by silting, nutrient pollution, drainage, and invasive species. In Britain, only the marl lakes of the more remote parts of northern Scotland are likely to remain pristine into the near future.[38]
See also
- Chemistry:Agricultural lime – Soil additive containing calcium carbonate and other ingredients
- Earth:Keuper marl
References
Citations
- ↑ Boggs 2006, p. 172.
- ↑ Pettijohn (1957), pp. 368–369.
- ↑ Blatt & Tracy 1996, p. 217.
- ↑ 4.0 4.1 Pettijohn (1957), pp. 410–411.
- ↑ Perri, Dominici & Critelli (2015).
- ↑ Nesse 2000, p. 249.
- ↑ Bristow, Mortimore & Wood 1997.
- ↑ Lauridsen & Surlyk 2008.
- ↑ Harris 1996, p. 57.
- ↑ Krijgsman (2001).
- ↑ Murphy & Wilkinson (1980).
- ↑ 13.0 13.1 13.2 Parker 2005.
- ↑ Wiik et al. (2015b).
- ↑ Leeder 2011, p. 663.
- ↑ Görög 2007.
- ↑ 17.0 17.1 Warncke 2015.
- ↑ 18.0 18.1 18.2 18.3 Jackson 1997, "marl lake".
- ↑ Mathew 1993.
- ↑ 20.0 20.1 Winiwarter & Blum 2008.
- ↑ 21.0 21.1 21.2 Dodgshon 1978.
- ↑ Frossard et al. 2009.
- ↑ Shannon 2020.
- ↑ Sheridan 1979.
- ↑ Geological Survey of New Jersey (1880), p. 184.
- ↑ Woll (1896), p. 295.
- ↑ New Jersey State Centennial Board (1877), p. 203.
- ↑ Geological Survey of New Jersey (1887).
- ↑ 29.0 29.1 Morse & Smith 2011.
- ↑ Rakhimov et al. 2017.
- ↑ Soltani, Tarighat & Varmazyari 2018.
- ↑ Rakhimova et al. 2018.
- ↑ Rankin & Williams 2012.
- ↑ Miščević 2020.
- ↑ Bahadori, Hasheminezhad & Taghizadeh 2019.
- ↑ Pearson & Scholtis 2021.
- ↑ Duston, Owen & Wilkinson 1986.
- ↑ 38.0 38.1 Pentecost 2009.
- ↑ EPA Catchments Unit 2020.
Bibliography
- New Jersey State Centennial Board (1877). Report of the New Jersey Commissioners on the Centennial Exhibition. Naar, Day, & Naar, printers. p. 203. https://archive.org/details/reportnewjersey00boargoog. Retrieved 2017-01-06.
- Geological Survey of New Jersey (1880). Annual Report of the State Geologist. p. 184. https://archive.org/details/annualreportsta04goog. Retrieved 2017-01-06.
- Geological Survey of New Jersey (1887). Annual Report of the State Geologist. pp. 193–. https://books.google.com/books?id=k2XyAAAAMAAJ&pg=PA193.
- Woll, F. W. (1896). "The Marls of Wisconsin". Thirteenth Annual Report of the Agricultural Experiment Station of the University of Wisconsin. 13. Madison, WI: Democrat Printing Company. p. 295. https://books.google.com/books?id=FAYTAAAAYAAJ&pg=PA295. Retrieved 2017-01-06.
- Bahadori, Hadi; Hasheminezhad, Araz; Taghizadeh, Farshad (February 2019). "Experimental Study on Marl Soil Stabilization Using Natural Pozzolans". Journal of Materials in Civil Engineering 31 (2): 04018363. doi:10.1061/(ASCE)MT.1943-5533.0002577.
- Blatt, Harvey; Tracy, Robert J. (1996). Petrology : igneous, sedimentary, and metamorphic. (2nd ed.). New York: W.H. Freeman. ISBN 0716724383.
- Boggs, Sam (2006). Principles of sedimentology and stratigraphy (4th ed.). Upper Saddle River, N.J.: Pearson Prentice Hall. p. 172. ISBN 0131547283.
- Bristow, Roger; Mortimore, Rory; Wood, Christopher (January 1997). "Lithostratigraphy for mapping the Chalk of southern England". Proceedings of the Geologists' Association 108 (4): 293–315. doi:10.1016/S0016-7878(97)80014-4. Bibcode: 1997PrGA..108..293B.
- Dodgshon, Robert A. (1978). "Land Improvement in Scottish Farming: Marl and Lime in Roxburghshire and Berwickshire in the Eighteenth Century". The Agricultural History Review 26 (1): 1–14.
- Duston, Nina M.; Owen, Robert M.; Wilkinson, Bruce H. (December 1986). "Water chemistry and sedimentological observations in littlefield lake, michigan: Implications for lacustrine marl deposition". Environmental Geology and Water Sciences 8 (4): 229–236. doi:10.1007/BF02524950. Bibcode: 1986EnGeo...8..229D.
- Harris, C.S., ed (1996). Engineering Geology of the Channel Tunnel. London: Thomas Telford. p. 57. ISBN 0-7277-2045-7.
- EPA Catchments Unit (28 January 2020). "Lough Carra marl lake - protecting one of Ireland's most unique and threatened habitats". Environmental Protection Agency. https://www.catchments.ie/lough-carra-marl-lake-protecting-one-of-irelands-most-unique-and-threatened-habitats/.
- Frossard, E.; Bünemann, E.; Jansa, J.; Oberson, A.; Feller, C. (2009). "Concepts and practices of nutrient management in agro-ecosystems: Can we draw lessons from history to design future sustainable agricultural production systems". Die Bodenkultur 60 (1): 43–60. https://diebodenkultur.boku.ac.at/volltexte/band-60/heft-1/frossard.pdf. Retrieved 19 March 2022.
- Görög, Péter (September 2007). "Characterization and mechanical properties of the Eocene Buda Marl". Central European Geology 50 (3): 241–258. doi:10.1556/CEuGeol.50.2007.3.4. Bibcode: 2007CEJGl..50..241G. https://www.researchgate.net/publication/250010203.
- Jackson, Julia A., ed (1997). Glossary of geology. (Fourth ed.). Alexandria, Virginia: American Geological Institute. ISBN 0922152349.
- Krijgsman, W. (2001). "Astrochronology for the Messinian Sorbas basin (SE Spain) and orbital (precessional) forcing for evaporite cyclicity". Sedimentary Geology 140 (1–2): 43–60. doi:10.1016/S0037-0738(00)00171-8. Bibcode: 2001SedG..140...43K. https://dspace.library.uu.nl/bitstream/1874/1632/1/Krijgsman01.pdf.
- Lauridsen, B.W.; Surlyk, F. (November 2008). "Benthic faunal response to late Maastrichtian chalk–marl cyclicity at Rørdal, Denmark". Palaeogeography, Palaeoclimatology, Palaeoecology 269 (1–2): 38–53. doi:10.1016/j.palaeo.2008.07.001. Bibcode: 2008PPP...269...38L.
- Leeder, M. R. (2011). Sedimentology and sedimentary basins : from turbulence to tectonics (2nd ed.). Chichester, West Sussex, UK: Wiley-Blackwell. ISBN 9781405177832.
- Mathew, W. M. (1993). "Marling in British Agriculture: A Case of Partial Identity". The Agricultural History Review 41 (2): 97–110.
- Miščević, P. (2020). "Effect of drying and wetting on mechanical characteristics of Eocene flysch marl". Geotechnical hazards. Boca Raton. pp. 737–741. doi:10.1201/9781003078173-99. ISBN 9781003078173. https://www.taylorfrancis.com/chapters/edit/10.1201/9781003078173-99/effect-drying-wetting-mechanical-characteristics-eocene-flysch-marl-mi%C5%A1%C4%8Devi%C4%87. Retrieved 1 February 2022.
- Morse, David; Smith, Michael (2011). "Marl in the Coastal Plain of North Carolina: From Agriculture to Aquaculture". Geological Society of America Abstracts with Programs 43 (2): 8. https://www.researchgate.net/publication/298971329. Retrieved 22 December 2020.
- Murphy, David H.; Wilkinson, Bruce H. (April 1980). "Carbonate deposition and facies distribution in a central Michigan marl lake". Sedimentology 27 (2): 123–135. doi:10.1111/j.1365-3091.1980.tb01164.x. Bibcode: 1980Sedim..27..123M.
- Nesse, William D. (2000). Introduction to mineralogy. New York: Oxford University Press. ISBN 9780195106916.
- Nourmohamadi, Mohammad Sadi; Abdula, Rzger A.; Albeyati, Fawzi; Sharezwri, Arkan O.; Perot, Edres M.; Jassim, Shamsadin E.; Othman, Nechirvan H. (30 November 2020). "Green Glauconitic Marl Bed As A Sequence Stratigraphical Key For Interpretation Of Contact Between Qamchuqa And Bekhme Formations In Bekhal Area, Kurdistan Region, NE Iraq". Bulletin of the Geological Society of Malaysia 70 (1): 29–38. doi:10.7186/bg70202003.
- Parker, Alan (24 July 2005). "There's Marl in Them Thar Ponds". Northern Woodlands (Center for Northern Woodlands Education). https://northernwoodlands.org/outside_story/article/theres-marl-in-them-thar-ponds.
- Pearson, F.J.; Scholtis, A. (2021). "Controls on the chemistry of pore water in a marl of very low permeability". Water-rock interaction : proceedings of the 8th International Symposium, WRI-8, Vladivostok, Russia, 15-19 August 1995 (1st ed.). London. doi:10.1201/9780203734049-8. ISBN 9780203734049. https://www.taylorfrancis.com/chapters/edit/10.1201/9780203734049-8/controls-chemistry-pore-water-marl-low-permeability-pearson-andreas-scholtis. Retrieved 1 February 2022.
- Pentecost, Allan (December 2009). "The Marl Lakes of the British Isles". Freshwater Reviews 2 (2): 167–197. doi:10.1608/FRJ-2.2.4.
- Perri, Francesco; Dominici, Rocco; Critelli, Salvatore (March 2015). "Stratigraphy, composition and provenance of argillaceous marls from the Calcare di Base Formation, Rossano Basin (northeastern Calabria)". Geological Magazine 152 (2): 193–209. doi:10.1017/S0016756814000089. Bibcode: 2015GeoM..152..193P.
- Pettijohn, F. J. (1957). Sedimentary Rocks (2nd ed.). New York: Harper & Brothers. OCLC 551748. https://archive.org/details/sedimentaryrocks00pett.
- Rakhimov, Ravil Z.; Rakhimova, Nailia R.; Gaifullin, Albert R.; Morozov, Vladimir P. (May 2017). "Properties of Portland cement pastes enriched with addition of calcined marl". Journal of Building Engineering 11: 30–36. doi:10.1016/j.jobe.2017.03.007.
- Rakhimova, Nailia R.; Rakhimov, Ravil Z.; Morozov, Vladimir P.; Gaifullin, Albert R.; Potapova, Ludmila I.; Gubaidullina, Alfiya M.; Osin, Yury N. (July 2018). "Marl-based geopolymers incorporated with limestone: A feasibility study". Journal of Non-Crystalline Solids 492: 1–10. doi:10.1016/j.jnoncrysol.2018.04.015. Bibcode: 2018JNCS..492....1R.
- Rankin, Bill; Williams, Ron (2012). "Channel Tunnel". The Geological Society of London. https://www.geolsoc.org.uk/GeositesChannelTunnel.
- Shannon, W.D. (2020). "'An excellent improver of the soil': Marl and the landscape of lowland Lancashire". Agricultural History Review 68 (2): 141–167. https://www.ingentaconnect.com/content/bahs/agrev/2020/00000068/00000002/art00003. Retrieved 19 March 2022.
- Sheridan, Richard C. (1979). "Chemical Fertilizers in Southern Agriculture". Agricultural History 53 (1): 308–18.
- Soltani, Abolfazl; Tarighat, Amir; Varmazyari, Masoud (November 2018). "Calcined Marl and Condensed Silica Fume as Partial Replacement for Ordinary Portland Cement". International Journal of Civil Engineering 16 (11): 1549–1559. doi:10.1007/s40999-018-0289-9.
- Warncke, Darryl (10 November 2015). "Lime for Michigan Soils". Michigan State University. https://www.canr.msu.edu/resources/lime_for_michigan_soils_e0471.
- Wiik, Emma; Bennion, Helen; Sayer, Carl D.; Davidson, Thomas A.; Clarke, Stewart J.; McGowan, Suzanne; Prentice, Stephen; Simpson, Gavin L. et al. (12 August 2015a). "The coming and going of a marl lake: multi-indicator palaeolimnology reveals abrupt ecological change and alternative views of reference conditions". Frontiers in Ecology and Evolution 3. doi:10.3389/fevo.2015.00082.
- Wiik, Emma; Bennion, Helen; Sayer, Carl D.; Davidson, Thomas A.; McGowan, Suzanne; Patmore, Ian R.; Clarke, Stewart J. (November 2015b). "Ecological sensitivity of marl lakes to nutrient enrichment: evidence from Hawes Water, UK" (in en). Freshwater Biology 60 (11): 2226–2247. doi:10.1111/fwb.12650.
- Winiwarter, Verena; Blum, Winfried E. H. (June 2008). "From marl to rock powder: On the history of soil fertility management by rock materials". Journal of Plant Nutrition and Soil Science 171 (3): 316–324. doi:10.1002/jpln.200625070.
Further reading
- Schurrenberger, D., Russell, J. and Kerry Kelts. 2003. Classification of lacustrine sediments based on sedimentary components. Journal of Paleolimnology 29: 141–154.
External links
- Chalk of Kent by C. S. Harris
- Palaeoenvironmental Interpretation of the Early Postglacial Sedimentary Record of a Marl Lake
 |