Chemistry:Totarol

| |
| Names | |
|---|---|
| IUPAC name
14-(Propan-2-yl)podocarpa-8,11,13-trien-13-ol
| |
| Systematic IUPAC name
(4bS,8aS)-4b,8,8-Trimethyl-1-(propan-2-yl)-4b,5,6,7,8,8a,9,10-octahydrophenanthren-2-ol | |
| Other names
(4bS)-trans-8,8-Trimethyl-4b,5,6,7,8,8a,9,10-octahydro-1-isopropylphenanthren-2-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H30O | |
| Molar mass | 286.459 g·mol−1 |
| Melting point | 128 to 132 °C (262 to 270 °F; 401 to 405 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Totarol is a naturally produced diterpene that is bioactive as totarol. It was first isolated by McDowell and Easterfield from the heartwood of Podocarpus totara, a conifer tree found in New Zealand.[2] Podocarpus totara was investigated for unique molecules due to the tree's increased resistance to rotting.[2] Recent studies have confirmed totarol's unique antimicrobial and therapeutic properties. Consequently, totarol is a candidate for a new source of drugs and has been the goal of numerous syntheses.
Discovery
Totarol was discovered in 1910[3] by New Zealand scientist Sir Thomas Hill Easterfield.[4] While investigating the properties of Miro, Kahikatea, Rimu, Matai and Totara, Easterfield detected a "crystalline bloom" on totara boards a few hours after leaving the planing machine. After extraction of totarol from Podocarpus totara, Easterfield observed no other compound had been cited in chemical literature before with this formula. Easterfield and his colleague J.C McDowell proposed the name “totarol” in a follow-up paper in 1915,[5] as the crystalline substance was believed to possess a tertiary alcohol group. In 1937 Short and Stromberg continued investigations, publishing Totarol Part 1.[2] In 1951 Short and Wang became the first to identify the chemical structure of totarol with their paper Totarol Part 2.[6]
Occurrence
Although totarol was first isolated in Podocarpus totara, totarol has also been identified in numerous other species of Podocarpaceae and Cupressaceae, with the majority found in the genus Podocarpus of the family Podocarpaceae and the subfamily Cupressoideae of the family Cupressaceae.[7] Outside Podocarpus and Cupressoideae, totarol is rarely found in the plant kingdom.[8] However, totarol has recently been isolated in Rosmarinus officinalis (rosemary).[9] The gymnosperms that contain totarol are distributed worldwide but are concentrated in North America, the far-south regions of South America, East Asia and East Africa.[10]
Biological activity
Antimicrobial activity
Totarol motivates research in drug discovery due to its ability to inhibit numerous microorganisms. Totarol exhibits antimicrobial properties in numerous species including gram-positive bacteria, acid-fast bacteria, nematodes, parasitic protozoans, crustaceous foulers (Table 1). In addition to inhibiting microorganisms by itself, totarol exhibits inhibitory synergy with currently used antimicrobial drugs: totarol potentiates isonicotinic acid hydrazide against various Mycobacteria.;[11] methicillin against Mycobacterium tuberculosis and Staphylococcus aureus;[12] and anacardic acid[13] and erythromycin[14] against Staphylococcus aureus. In nature, totarol is a key player in gymnosperm's defense against harmful microbes: gymnosperms that produce totarol are resistant to rotting.
Table 1. Antibacterial activity of totarol against microorganisms
| Microorganism | MIC (μg/ml) | IC50(μg/ml) |
|---|---|---|
| Artemia salina[15] | -
|
1
|
| Bacterium ammoniagenes[13] | 0.78
|
-
|
| Bacillus subtilis[13] | 1.56
|
-
|
| Caenorhabditis elegans[15] | -
|
80
|
| Enterococcus faecalis[16] | 2
|
-
|
| Klebsiella pneumoniae[16] | >32
|
-
|
| Mycobacterium aurum[17] | 2
|
7.5
|
| Mycobacterium fortuitum[17] | 4
|
7.5
|
| Mycobacterium phlei[17] | 4
|
7.5
|
| Mycobacterium smegmatis[17] | 2
|
7.5
|
| Mycobacterium tuberculosis H37Rv[17] | 21.1
|
7.5
|
| Leishmania donovani[13] | -
|
3.5
|
| Proprionibacterium acnes[18] | 3.9
|
-
|
| Staphylococcus aureus ATCC 12598[18] | 1.56
|
-
|
| Staphylococcus aureus ATCC 33591[18] | 0.78
|
-
|
| Staphylococcus aureus ATCC 11632[18] | 0.78
|
-
|
| Streptococcus mutans[13] | 0.78
|
-
|
| Streptococcus pneumoniae[16] | 2
|
-
|
Mechanism of antimicrobial inhibition
Although totarol exhibits antimicrobial properties, the mode of action is unclear and various methods of inhibitory action have been proposed. In Staphylococcus aureus strains resistant to penicillin via creation of penicillin binding protein 2’ (PBP2’), totarol may inhibit the synthesis of PBP2’.[12] Totarol may inhibit effluxing Staphylococcus aureus strains through inhibition of MsrA, although it is unclear if MsrA is an efflux pump.[14] Totarol may also gain its antibacterial properties by inhibiting bacterial respiratory transport[19] but this is very unlikely because totarol is also effective against anaerobic organisms.[20] Recently totarol was also hypothesized to inhibit gram-positive and acid-fast bacteria via inhibition of FtsZ protein, which forms the Z-ring, a polymer necessary for efficient bacterial cell cytokinesis.[21]
Totarol may also function by disrupting the structural integrity of the phospholipid bilayer of bacteria by weakening Van der Waals interactions with its phenolic group,[22][23][24] which also results in bacterial cells unable to synthesize ATP.[25] Motivation for totarol functioning via disruption of membrane structure is due to its high phospholipid/water partition coefficient.[23] However, totarol's partitioning capability was only observed at concentrations 10 to 100 fold higher than required for antibacterial activity.[25] Thus it is unlikely that totarol is an uncoupler of bacterial respiration at the low levels observed in antimicrobial studies.
Traditional use
The use of Podocarpus totara extract in Maori medicines for treatment of fevers, asthma, coughs, cholera, distemper, chest complaints and venereal disease dates back to over 100 years.[26] The timber of Podocarpus totara is renowned for its resilience against rotting, which made it valuable to Maori for housing, waka (canoes), fencing, pa stockades, drinking vessels, shovels and carvings in chiefs houses. Totara bark was used to cover kelp bags used for preserved muttonbirds.[27] European settlers of New Zealand used the wood for wharf piles, bridges, railway sleepers, telegraph poles, lighthouses, mining equipment, fence posts and foundation blocks. The durability stems from the anti-bacterial activity of totarol. Houses, churches, grave markers, and even cobbles and kerbs were made of totara; strips of the bark were used as a roofing material.[28] The presence of totarol means totara wood resists decay and insect attack in the heart timber.[29] Totarol has been found in organic matter in the embankments of a neolithic site in Northern Sweden. Totarol from Cupressaceae resin was possibly used for its antibacterial and antifungal properties to preserve meat, as well as for its ability to repel insects.[30] Despite totarol's antimicrobial potential, its commercial use is currently limited to cosmetic purposes. For totarol to be used clinically, its mode of action needs to be clearly defined.
Biochemical properties
Totarol decreases the plasma levels of estrogens[31] and can also effectively reduce pathogenic hepatic cells in vitro.[32] Totarol's anti-cancer activity is hypothesized to be due to the natural product's ability to form an o-quinone methide in vivo.[33] Totarol also prevents cells from undergoing oxidative stress in vitro by acting as a hydrogen donor to peroxy radicals or reacting with other peroxy radicals to terminate undesirable radical reactions.[19]
Biosynthesis
Totarol is a precursor to the formation of nagilactones[34] that possess antifungal properties not possessed by totarol.[19][35] Consequently, gymnosperms that produce totarol and nagilactones are able to defend themselves against bacteria and fungi.
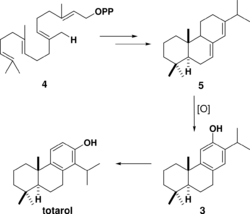
The biosynthesis of totarol was difficult to determine. The main reason for the challenge in determining how the secondary metabolite is produced is because totarol does not follow the isoprene rule: the isopropyl group of totarol is in the “wrong” place[9] at C14. Initially, it was hypothesized that totarol and the “normal” diterpene ferruginol, also found in Podocarpaceae, were derived by a precursor 2 that would be dehydrated and have its isopropyl group migrate to produce totarol 1 and ferruginol 3 (Scheme 1).[36] This hypothesis was motivated by the well known santonin-desmotroposantonin rearrangement of steroid dienones into aromatic compounds. It is now accepted that totarol is synthesized biologically from ferruginol.[9] Geranyl geranyl pyrophosphate 4 undergoes typical diterpene cyclization to form (−)-abietadiene 5, which is oxidized to form ferruginol 3, which proceeds through a spiro intermediate to form totarol (Scheme 2).
Synthesis
Totarol has been the subject of numerous syntheses. The first total synthesis of totarol (Scheme 3)[37] utilized 6 and the alkyne 7 to yield 8 which was converted to the corresponding ketone 9 via hydrogenation followed by cyclization with polyphosphoric acid. 9 was subsequently converted to 10 and another ketone that were inseparable by chromatography. The synthesis was finalized by treatment with N-Bromosuccinimide followed by debromination to yield (±)-totarol. The main downfall of this synthesis was that in multiple steps, complete conversion of reactant to products was not observed and undesirable side products were often not separable by chromatography. However, since this was the first total synthesis of (±)-totarol, it is notable.
Total enantioselective synthesis
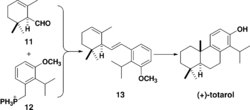
The first total enantioselective synthesis of totarol was achieved in 1979 (Scheme 4).[38] The key step in the synthesis is the formation of 13 via a Wittig reaction between 11 and 12. This same cyclization can also be achieved via a Friedel-Crafts alkylation and cyclization.[39] Subsequent hydrogenation of 13 followed by intramolecular cyclization with aluminium chloride forms the B ring and totarylmethyl ether which is demethylated by boron tribromide to yield totarol.
A more recent organic synthesis of totarol was achieved by utilizing 14, a lamdane diterpene named zamoranic acid (Scheme 5).[40] The addition of the isopropyl group in the chemical synthesis was achieved with complete stereoselectivity. Acetylation to yield 15 required high temperatures due to the steric hindrance of the isopropyl group. Cis-hydroxylation followed by cleavage with H5IO6 yielded a diol that was acylated in pyridine and oxidized to give 16. The key step in the synthesis was the cyclization of ring C: 16 was treated with SmI2 to yield totarane diastereomers which were separated by column chromatography. The desired diastereomer was treated with p-TsOH in benzene to yield 17. The synthesis was completed by a halogenation-dehydrogenation sequence and subsequent bromination to yield 18 and ring aromatization with elimination via a lithium complex.
Total chemoenzymatic synthesis
Chemoenzymatic synthesis of totarol has also been achieved with high yield (41.8%) (Scheme 6).[41] A racemic beta-keto ester 19 undergoes lipase-assisted resolution to yield chiral alcohol 20. Treatment of 20 with 10% HCl and p-TsOH gives αβ-unsaturated ketone 21. A Michael addition with the anion obtained from the reaction of methyl 5-methyl-3-oxohexanoate 13 with NaOMe gives a 2:1 diastereomeric mixture of 22 which is hydrolyzed to yield 23 which is brominated and debrominated to yield totarol.
Research
| Year | Subject |
|---|---|
| 1992 | Bacteria killer and antibiotic enhancer[42] |
| 1996 | Anti methicillin-resistant Staphylococcus aureus[43] |
| 1997 | Antioxidant[44] |
| 1998 | Diterpene[45] |
| 1999 | Antibacterial[46] |
| 1999 | Anti methicillin-resistant Staphylococcus aureus[47] |
| 2000 | Mosquito insecticide[48] |
| 2001 | Tuberculosis[49] |
| 2001 | Cell change[50] |
| 2002 | Insect repellant[51] |
| 2003 | Antiplasmodial and cytotoxic[52] |
| 2004 | Antimalarial[53] |
| 2005 | Anti inflammatory[54] |
| 2006 | Anti acne[55] |
| 2007 | |
| 2007 | Staphyloccocus auereus inhibition[56] |
| 2007 | Tuberculosis[57] |
| 2015 | Neurological disorders[58] |
| 2015 | Bacillus subtilis alteration[59] |
| 2017 | Ultrasound treatment on whey protein-totarol nanoparticles[60] |
| 2017 | Surgical site infection[61] |
| 2018 | Plant defense[62] |
| 2018 | Food preservative[63] |
| 2019 | Historical food preservative[30] |
| 2019 | Antibacterial for dental implants[64] |
| 2020 | Whey protein based tissue adhesive[65] |
| 2020 | Mastitis[66] |
| 2021 | Anticancer[67] |
| 2022 | Drug delivery destroys microbial biofilm[68] |
| 2022 | Anti inflammatory[69] |
| 2023 | |
| 2023 |
Extraction
Totarol is extracted by supercritical extraction. The process uses high pressure carbon dioxide under specific temperature, pressure and gas flow conditions to extract totarol from powdered totara wood. Totarol can be extracted from dead wood, negating the need to cut down live trees. Although totarol can be extracted from other Podocarpus, some trees in the cypress family (cypress, juniper, thuja) and from rosemary, it is most abundant in Podocarpus totara. [70]
Products
Products for sale containing totarol include toothpastes, tooth tablets, mouthwash, toners, cleansers, moisturisers, face masks, concealers, blemish control, anti-acne, pimple patches, face cream, eye cream, sun screen, deodorants, face mists, facial wash, anti-wrinkle, restorative serum, renew cream, scar removal serum, dandruff control, scalp treatment, shampoo bar, pet skin cream, hand wash, hand cream, pressed powder makeup, mascara, rescue cream, skin whitener, lip tint, pregnancy body oil, mouth freshener, sore throat relief, cold and flu nasal spray.[71]
Other uses
Totarol may also be used as an indicator for the quality of juniper berry based spirits. Juniper berries that contain diterpenoids including totarol are used for the aromatization and production of some gins. Consequently, totarol can aid in the characterization of different types of gin or commercial brands, vouching for the authenticity and quality of the product.[72]
Totarol has been found on the posterior tibia of Frieseomelitta silvestrii languida, a species of stingless bees from Brazil. Frieseomelitta silvestrii languida collect resin to create a protective barrier around the opening of their nest to ward off insects from settling near the nest's entrance.[73] The presence of totarol can aid in the determination of this bee species.
References
- ↑ "(4bS)-trans-8,8-Trimethyl-4b,5,6,7,8,8a,9,10-octahydro-1-isopropylphenanthren-2-ol". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/ProductDetail.do?N4=532657.
- ↑ 2.0 2.1 2.2 "116. Totarol. Part I.". Journal of the Chemical Society (Resumed): 516–520. 1937. doi:10.1039/JR9370000516.
- ↑ "Totarol: a Non-Conventional Diterpenoid". Australian Journal of Chemistry 48 (5): 883. 1995. doi:10.1071/ch9950883. ISSN 0004-9425.
- ↑ "Studies on the chemistry of the New Zealand flora. Part IV.—The chemistry of the Podocarpi.". Transactions and Proceedings of the New Zealand Institute 43: 53–55. 1910. https://paperspast.natlib.govt.nz/imageserver/periodicals/P29pZD1UUFJTTloxOTEwLTQzLjIuMi4xLjgmZ2V0cGRmPXRydWU=.
- ↑ "The chemistry of Podocarpus totara and Podocarpus spicatus.". Transactions of the New Zealand Institute 48: 518–520. 1915. https://paperspast.natlib.govt.nz/imageserver/periodicals/P29pZD1UUFJTTloxOTE1LTQ4LjIuNS4xLjU2JmdldHBkZj10cnVl.
- ↑ "662. Totarol. Part II". Journal of the Chemical Society (Resumed): 2979–2987. 1951. doi:10.1039/jr9510002979. ISSN 0368-1769. https://pubs.rsc.org/en/content/articlelanding/1951/jr/jr9510002979.
- ↑ "Totarol, totaradiol and ferruginol: three diterpenes from Thuja plicata (Cupressaceae)". Biochemical Systematics and Ecology 29 (2): 215–217. February 2001. doi:10.1016/s0305-1978(00)00047-8. PMID 11106853.
- ↑ "An unprecedented condensation pathway leading to the formation of phenolic C40 bis-diterpenoids in sediments from the Lower Oligocene of the Rhine Rift Valley.". Organic Geochemistry 39 (6): 658–675. June 2008. doi:10.1016/j.orggeochem.2008.02.020. Bibcode: 2008OrGeo..39..658L.
- ↑ 9.0 9.1 9.2 "Totarol: A non-conventional diterpenoid.". Australian Journal of Chemistry 48 (5): 883–917. 1995. doi:10.1071/CH9950883.
- ↑ World Checklist and Bibliography of Conifers (2nd ed.). Kew: Royal Botanic Gardens. 2001. p. 212.
- ↑ "Antimycobacterial constituents from Juniperus procera, Ferula communis and Plumbago zeylanica and their in vitro synergistic activity with isonicotinic acid hydrazide". Phytotherapy Research 18 (11): 934–7. November 2004. doi:10.1002/ptr.1420. PMID 15597311.
- ↑ 12.0 12.1 "Plants as a source of bacterial resistance modulators and anti-infective agents.". Phytochemistry Reviews 4 (1): 63–78. January 2005. doi:10.1007/s11101-005-2494-9. Bibcode: 2005PChRv...4...63G.
- ↑ 13.0 13.1 13.2 13.3 13.4 "Antibacterial activity of totarol and its potentiation". Journal of Natural Products 55 (10): 1436–40. October 1992. doi:10.1021/np50088a008. PMID 1453180.
- ↑ 14.0 14.1 "The phenolic diterpene totarol inhibits multidrug efflux pump activity in Staphylococcus aureus". Antimicrobial Agents and Chemotherapy 51 (12): 4480–3. December 2007. doi:10.1128/AAC.00216-07. PMID 17664318.
- ↑ 15.0 15.1 "Antiparasitic, nematicidal and antifouling constituents from Juniperus berries". Phytotherapy Research 22 (12): 1570–1576. December 2008. doi:10.1002/ptr.2460. PMID 19067375.
- ↑ 16.0 16.1 16.2 "The synthesis and antibacterial activity of totarol derivatives. Part 1: modifications of ring-C and pro-drugs". Bioorganic & Medicinal Chemistry 7 (9): 1953–1964. September 1999. doi:10.1016/s0968-0896(99)00162-5. PMID 10530944.
- ↑ 17.0 17.1 17.2 17.3 17.4 "Antimycobacterial terpenoids from Juniperus communis L. (Cuppressaceae)". Journal of Ethnopharmacology 126 (3): 500–5. December 2009. doi:10.1016/j.jep.2009.09.007. PMID 19755141.
- ↑ 18.0 18.1 18.2 18.3 "Antibacterial activity of anacardic acid and totarol, alone and in combination with methicillin, against methicillin-resistant Staphylococcus aureus". The Journal of Applied Bacteriology 80 (4): 387–94. April 1996. doi:10.1111/j.1365-2672.1996.tb03233.x. PMID 8849640.
- ↑ 19.0 19.1 19.2 "Inhibition of lipid peroxidation by diterpenoid from Podocarpus nagi". Experientia 52 (6): 573–6. June 1996. doi:10.1007/BF01969731. PMID 8698092.
- ↑ "Inhibition of oral bacteria by phenolic compounds. Part 1. QSAR analysis using molecular connectivity.". Quantitative Structure-Activity Relationships 17 (4): 327–337. August 1998. doi:10.1002/(SICI)1521-3838(199808)17:04<327::AID-QSAR327>3.0.CO;2-O.
- ↑ "Totarol inhibits bacterial cytokinesis by perturbing the assembly dynamics of FtsZ". Biochemistry 46 (14): 4211–20. April 2007. doi:10.1021/bi602573e. PMID 17348691.
- ↑ "Effects of (+)-totarol, a diterpenoid antibacterial agent, on phospholipid model membranes". Biochimica et Biophysica Acta (BBA) - Biomembranes 1511 (2): 281–90. April 2001. doi:10.1016/s0005-2736(01)00284-x. PMID 11286971.
- ↑ 23.0 23.1 "A fluorescence study of the interaction and location of (+)-totarol, a diterpenoid bioactive molecule, in model membranes". Biochimica et Biophysica Acta (BBA) - Biomembranes 1509 (1–2): 167–175. December 2000. doi:10.1016/s0005-2736(00)00291-1. PMID 11118528.
- ↑ "A MAS-NMR study of the location of (+)-totarol, a diterpenoid bioactive molecule, in phospholipid model membranes". Chemistry and Physics of Lipids 119 (1–2): 33–9. October 2002. doi:10.1016/s0009-3084(02)00050-6. PMID 12270671.
- ↑ 25.0 25.1 "The synthesis and antibacterial activity of totarol derivatives. Part 3: Modification of ring-B". Bioorganic & Medicinal Chemistry 8 (7): 1663–75. July 2000. doi:10.1016/s0968-0896(00)00096-1. PMID 10976514.
- ↑ "Ethnobotany, phytochemistry and pharmacology of Podocarpus sensu latissimo (sl).". South African Journal of Botany 76 (1): 1–24. January 2010. doi:10.1016/j.sajb.2009.09.002.
- ↑ "Podocarpus Totara". July 1, 2020. https://rauropiwhakaoranga.landcareresearch.co.nz/names/fb454c7e-cb0c-4d98-9f97-1e9d14d3a080.
- ↑ Gilchrist, Shane (July 24, 2017). "Children of Tane". https://www.odt.co.nz/lifestyle/magazine/children-tane.
- ↑ Duncan, C R (2004-01-01). "Fire protection of New Zealand's traditional Maori buildings". https://d39d3mj7qio96p.cloudfront.net/media/documents/SR128_Fire_protection_of_New_Zealands_traditional_Maori_buildings.pdf.
- ↑ 30.0 30.1 "Fire, meat and totarol: organic matter in the embankments of the Neolithic site Bastuloken (North Sweden)". Analytical Pyrolysis Letters APL007: 1–16. https://pyrolyscience.com/wp-content/uploads/2019/09/APL007.pdf.
- ↑ "Aromatase inhibitory activities of standishinal and the diterpenoids from the bark of Thuja standishii". Planta Medica 68 (8): 742–5. August 2002. doi:10.1055/s-2002-33787. PMID 12221600.
- ↑ "Antifibrotic activity of diterpenes from Biota orientalis leaves on hepatic stellate cells". Archives of Pharmacal Research 31 (7): 866–71. July 2008. doi:10.1007/s12272-001-1239-9. PMID 18704328.
- ↑ "o-Quinone methides: intermediates underdeveloped and underutilized in organic synthesis.". Tetrahedron 58 (27): 5367–5406. July 2002. doi:10.1016/S0040-4020(02)00496-9.
- ↑ Bailly, Christian (2020-12-01). "Anticancer Activities and Mechanism of Action of Nagilactones, a Group of Terpenoid Lactones Isolated from Podocarpus Species" (in en). Natural Products and Bioprospecting 10 (6): 367–375. doi:10.1007/s13659-020-00268-8. ISSN 2192-2195. PMC 7648843. https://link.springer.com/10.1007/s13659-020-00268-8.
- ↑ "Antibacterial activity of long-chain alcohols against Streptococcus mutans.". Journal of Agricultural and Food Chemistry 41 (12): 2447–2450. December 1993. doi:10.1021/jf00036a045.
- ↑ "662. Totarol. Part II". Journal of the Chemical Society (Resumed): 2979–2987. 1951. doi:10.1039/JR9510002979.
- ↑ "520. Experiments on the synthesis of diterpenes. Part I. A total synthesis of (±)-totarol". Journal of the Chemical Society (Resumed): 2566–2572. 1958. doi:10.1039/JR9580002566.
- ↑ "The Total Synthesis of (+)-Totarol and (+)-Podototarin.". Bulletin of the Chemical Society of Japan 52 (5): 1450–1453. May 1979. doi:10.1246/bcsj.52.1450.
- ↑ "Stereocontrolled total synthesis of (±)-totaryl methyl ether and (±)-semperviryl methyl ether.". Tetrahedron 48 (41): 9101–9110. January 1992. doi:10.1016/S0040-4020(01)82004-4.
- ↑ "Synthesis of (+)-totarol.". Tetrahedron Letters 44 (49): 8831–8835. December 2003. doi:10.1016/j.tetlet.2003.09.193.
- ↑ "Chemoenzymatic synthesis of (+)-totarol,(+)-podototarin,(+)-sempervirol, and (+)-jolkinolides E and D.". Tetrahedron: Asymmetry 18 (24): 2915–2922. December 2007. doi:10.1016/j.tetasy.2007.11.024.
- ↑ "Antibacterial activity of totarol and its potentiation". Journal of Natural Products 55 (10): 1436–1440. October 1992. doi:10.1021/np50088a008. PMID 1453180.
- ↑ "Antibacterial activity of anacardic acid and totarol, alone and in combination with methicillin, against methicillin-resistant Staphylococcus aureus". The Journal of Applied Bacteriology 80 (4): 387–394. April 1996. doi:10.1111/j.1365-2672.1996.tb03233.x. PMID 8849640.
- ↑ Haraguchi, Hiroyuki; Ishikawa, Harumi; Kubo, Isao (1997-06-01). "Antioxidative Action of Diterpenoids from Podocarpus nagi" (in en). Planta Medica 63 (03): 213–215. doi:10.1055/s-2006-957655. ISSN 0032-0943. http://www.thieme-connect.de/DOI/DOI?10.1055/s-2006-957655.
- ↑ Nicolson, Kirsty (1998). Antibacterial properties of diterpenes and their derivatives: a thesis presented in partial fulfilment of the requirements for the degree of Master of Science in Microbiology at Massey University (Thesis thesis). Massey University.[1]
- ↑ "The synthesis and antibacterial activity of totarol derivatives. Part 1: modifications of ring-C and pro-drugs". Bioorganic & Medicinal Chemistry 7 (9): 1953–1964. September 1999. doi:10.1016/S0968-0896(99)00162-5. PMID 10530944.
- ↑ Nicolson, Kirsty; Evans, Gary; O'Toole, Paul W. (1999-10-01). "Potentiation of methicillin activity against methicillin-resistant Staphylococcus aureus by diterpenes" (in en). FEMS Microbiology Letters 179 (2): 233–239. doi:10.1111/j.1574-6968.1999.tb08733.x. https://academic.oup.com/femsle/article-lookup/doi/10.1111/j.1574-6968.1999.tb08733.x.
- ↑ "A Mosquito Larvicidal Diterpenoid Isolated from Podocarpus totara D. Don ex Lambert". Journal of Entomological Science 35 (4): 474–477. October 2000. doi:10.18474/0749-8004-35.4.474. ISSN 0749-8004. https://meridian.allenpress.com/jes/article-pdf/35/4/474/1562795/0749-8004-35_4_474.pdf.
- ↑ "Antimycobacterial terpenoids from Juniperus communis L. (Cuppressaceae)". Journal of Ethnopharmacology 126 (3): 500–505. December 2009. doi:10.1016/j.jep.2009.09.007. PMID 19755141.
- ↑ "Effects of (+)-totarol, a diterpenoid antibacterial agent, on phospholipid model membranes". Biochimica et Biophysica Acta (BBA) - Biomembranes 1511 (2): 281–290. April 2001. doi:10.1016/S0005-2736(01)00284-X. PMID 11286971.
- ↑ "The propolis of stingless bees: terpenes from the tibia of three Frieseomelitta species". Journal of Insect Physiology 48 (2): 249–254. February 2002. doi:10.1016/S0022-1910(01)00170-6. PMID 12770125.
- ↑ "Synthesis of totarol amino alcohol derivatives and their antiplasmodial activity and cytotoxicity". Bioorganic & Medicinal Chemistry 11 (20): 4417–4422. October 2003. doi:10.1016/S0968-0896(03)00491-7. PMID 13129578.
- ↑ Tacon C (2004). Chemical modification and pharmacological evaluation of the antimalarial natural product totarol (PDF) (Master Thesis thesis). University of Cape Town.
- ↑ , Gerard J."Method for treating skin disorders" patent US6881756B2, issued 2005-04-19
- ↑ "The use of totarol to treat acne in an adolescent: A case study". NZFP 33 (4): 253–255. August 15, 2006. https://www.essentiallynz.com/wp-content/images/Totarol_Case_Study.pdf.
- ↑ "The phenolic diterpene totarol inhibits multidrug efflux pump activity in Staphylococcus aureus". Antimicrobial Agents and Chemotherapy 51 (12): 4480–4483. December 2007. doi:10.1128/AAC.00216-07. PMID 17664318.
- ↑ "Totarol inhibits bacterial cytokinesis by perturbing the assembly dynamics of FtsZ". Biochemistry 46 (14): 4211–4220. April 2007. doi:10.1021/bi602573e. PMID 17348691.
- ↑ "Totarol prevents neuronal injury in vitro and ameliorates brain ischemic stroke: Potential roles of Akt activation and HO-1 induction". Toxicology and Applied Pharmacology 289 (2): 142–154. December 2015. doi:10.1016/j.taap.2015.10.001. PMID 26440581.
- ↑ "A comprehensive proteomic analysis of totarol induced alterations in Bacillus subtilis by multipronged quantitative proteomics". Journal of Proteomics 114: 247–262. January 2015. doi:10.1016/j.jprot.2014.10.025. PMID 25464363.
- ↑ "Effects of Ultrasound Treatment on Physiochemical Properties and Antimicrobial Activities of Whey Protein-Totarol Nanoparticles". Journal of Food Protection 80 (10): 1657–1665. October 2017. doi:10.4315/0362-028X.JFP-17-078. PMID 28876131.
- ↑ "Preventing Surgical Site Infections Using a Natural, Biodegradable, Antibacterial Coating on Surgical Sutures". Molecules 22 (9): 1570. September 2017. doi:10.3390/molecules22091570. PMID 28925959.
- ↑ "A Metabolomic and HPLC-MS/MS Analysis of the Foliar Phenolics, Flavonoids and Coumarins of the Fraxinus Species Resistant and Susceptible to Emerald Ash Borer". Molecules 23 (11): 2734. October 2018. doi:10.3390/molecules23112734. PMID 30360500.
- ↑ "Antibacterial activity and mode of action of totarol against Staphylococcus aureus in carrot juice". Journal of Food Science and Technology 55 (3): 924–934. March 2018. doi:10.1007/s13197-017-3000-2. PMID 29487434.
- ↑ "The application of natural antibacterial coating for the surface modification of dental implants and abutments" (in en). Clinical Oral Implants Research 30 (S19): 132. September 25, 2019. doi:10.1111/clr.90_13509. ISSN 0905-7161. https://onlinelibrary.wiley.com/doi/10.1111/clr.90_13509.
- ↑ "Formulation and Functional Properties of Whey Protein-Based Tissue Adhesive Using Totarol as an Antimicrobial Agent" (in en). Processes 8 (4): 496. 2020-04-24. doi:10.3390/pr8040496. ISSN 2227-9717.
- ↑ "Minimum Inhibitory and Bactericidal Concentrations of a Totarol™ formulation against Staphylococcus aureus strains obtained from bovine intramammary infections in New Zealand" (in en). Antimicrobials 2020. March 2020. doi:10.13140/RG.2.2.29327.33449. http://rgdoi.net/10.13140/RG.2.2.29327.33449.
- ↑ "Design, Hemiysnthesis, crystal structure and anticancer activity of 1, 2, 3-triazoles derivatives of totarol". Bioorganic Chemistry 115: 105165. October 2021. doi:10.1016/j.bioorg.2021.105165. PMID 34298240. https://hal.science/hal-03438882/file/Article%20totarol-triazole.pdf.
- ↑ "PLGA-Based Nanoplatforms in Drug Delivery for Inhibition and Destruction of Microbial Biofilm". Frontiers in Cellular and Infection Microbiology 12: 926363. 2022-06-21. doi:10.3389/fcimb.2022.926363. PMID 35800390.
- ↑ "Antimicrobial, toxicity, and anti-inflammatory activities of Buddleja perfoliata Kunth" (in en). Phytomedicine Plus 2 (4): 100357. October 13, 2022. doi:10.1016/j.phyplu.2022.100357.
- ↑ "Bioactive Totarol". July 23, 2023. https://www.totarol.com/.
- ↑ "Products with Totarol". INCIDecoder. https://incidecoder.com/ingredients/totarol.
- ↑ "Assessment of some diterpenoids in commercial distilled gin". Analytica Chimica Acta 628 (2): 222–9. November 2008. doi:10.1016/j.aca.2008.09.005. PMID 18929011.
- ↑ "The propolis of stingless bees: terpenes from the tibia of three Frieseomelitta species". Journal of Insect Physiology 48 (2): 249–254. February 2002. doi:10.1016/s0022-1910(01)00170-6. PMID 12770125.
 |