Chemistry:Titanocene pentasulfide
| |
| Names | |
|---|---|
| Other names
titanocene pentasulfide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C10H10S5Ti | |
| Molar mass | 338.382 |
| Appearance | red solid |
| Structure | |
| Dist. tetrahedral | |
| Related compounds | |
Related compounds
|
Zirconocene pentasulfide Titanocene dichloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
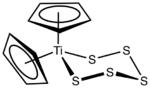
Titanocene pentasulfide is the organotitanium compound with the formula (C5H5)2TiS5, commonly abbreviated as Cp2TiS5. This metallocene exists as a bright red solid that is soluble in organic solvents. It is of academic interest as a precursor to unusual allotropes of elemental sulfur as well as some related inorganic rings.
Preparation and structure
Titanocene pentasulfide is prepared by treating Cp2TiCl2 with polysulfide salts:[1] It was first produced by the addition of elemental sulfur to titanocene dicarbonyl:[2]
- (C5H5)2Ti(CO)2 + 5⁄8 S8 → (C5H5)2TiS5 + 2 CO
The complex is viewed as a pseudotetrahedral complex of Ti(IV). The Ti–S distances are 2.420 and 2.446 Å and the S–S bond distances are of a normal range, 2.051–2.059 Å.[3] The molecule exhibits a dynamic NMR spectrum owing to the chair–chair equilibrium of the TiS5 ring which equivalizes the Cp signals at high temperatures.[4]
Reactions
Cp2TiS5 reacts with sulfur and selenium chlorides, ExCl2, to afford titanocene dichloride and various S5+x and S5Sex rings. Illustrative is the synthesis of S7 from disulfur dichloride:[5]
- (C5H5)2TiS5 + S2Cl2 → (C5H5)2TiCl2 + S7
It also reacts with alkenes and ketenes to give heterocycles composed of Ti, C and S. With trialkylphosphines, the cycle dimerize into rings of various sizes, depending on the trialkylphosphine used.[6]
References
- ↑ Shaver, Alan; McCall, James M.; Marmolejo, Gabriela (1990). "Cyclometallapolysulfanes (And Selanes) of Bis(η 5 ‐Cyclopentadienyl) Titanium(IV), Zirconium(IV), Molybdenum(IV), and Tungsten(IV)". Inorganic Syntheses. 27. 59–65. doi:10.1002/9780470132586.ch11. ISBN 9780470132586.
- ↑ "π-Complexes of Group IVA metals with cyclopentadiene, indene, and fluorine". Bull. Soc. Chim. France 11: 3548–64. 1966.
- ↑ Epstein, E. F.; Bernal, I. (1970). "Pentachalcogenide dianions in transition-metal complexes: crystal structure of bis-(π-cyclopentadienyl)titanium pentasulphide". J. Chem. Soc. D 1970 (7): 410–411. doi:10.1039/C29700000410.
- ↑ Shaver, Alan; McCall, James M. (1984). "Preparation and Variable-Temperature NMR Studies of the Metallacyclosulfanes Cp2MS5 and (MeSCp)MS3, Where M = Ti, Zr, and Hf". Organometallics 3 (12): 1823–1829. doi:10.1021/om00090a008.
- ↑ Steudel, Ralf; Eckert, Bodo (2003). "Solid Sulfur Allotropes Sulfur Allotropes". Topics in Current Chemistry 230: 1–80. doi:10.1007/b12110.
- ↑ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999). Advanced Inorganic Chemistry (6th ed.). Wiley. ISBN 978-0471199571.
 |


