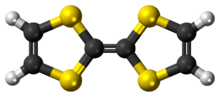
Chemistry:Tetrathiafulvalene

| |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2′-Bi(1,3-dithiolylidene) | |
| Other names
Δ2,2-Bi-1,3-dithiole
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1282106 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H4S4 | |
| Molar mass | 204.34 g·mol−1 |
| Appearance | Yellow solid |
| Melting point | 116 to 119 °C (241 to 246 °F; 389 to 392 K) |
| Boiling point | Decomposes |
| Insoluble | |
| Solubility in organic solvents | Soluble[vague] |
| Structure | |
| 0 D | |
| Hazards[1] | |
| Main hazards | combustible |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H317 | |
| P261, P280, P302+352, P333+313, P363, P501 | |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetrathiafulvalene (TTF) is an organosulfur compound with the formula (C
3H
2S
2)
2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, (C
5H
4)
2, by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives.[2]
Preparation
The high level of interest in TTFs has spawned the development of many syntheses of TTF and its analogues.[2] Most preparations entail the coupling of cyclic C
3S
2 building blocks such as 1,3-dithiole-2-thion or the related 1,3-dithiole-2-ones. For TTF itself, the synthesis begins with the cyclic trithiocarbonate H
2C
2S
2C=S (1,3-dithiole-2-thione), which is S-methylated and then reduced to give H
2C
2S
2CH(SCH
3) (1,3-dithiole-2-yl methyl thioether), which is treated as follows:[3]
- 2 [H
2C
2S
2CH]+
[BF
4]−
+ 2 N(CH
2CH
3)
3 → (H
2C
2S
2C)
2 + 2 [NH(CH
2CH
3)
3]+
[BF
4]−
Redox properties
Bulk TTF itself has unremarkable electrical properties. Distinctive properties are, however, associated with salts of its oxidized derivatives, such as salts derived from TTF+
.
The high electrical conductivity of TTF salts can be attributed to the following features of TTF:
- its planarity, which allows π-π stacking of its oxidized derivatives,
- its high symmetry, which promotes charge delocalization, thereby minimizing coulombic repulsions, and
- its ability to undergo oxidation at mild potentials to give a stable radical cation. Electrochemical measurements show that TTF can be oxidized twice reversibly:
Each dithiolylidene ring in TTF has 7π electrons: 2 for each sulfur atom, 1 for each sp2 carbon atom. Thus, oxidation converts each ring to an aromatic 6π-electron configuration, consequently leaving the central double bond essentially a single bond, as all π-electrons occupy ring orbitals.
History
The salt [TTF+]Cl− was reported to be a semiconductor in 1972.[5] Subsequently, the charge-transfer salt [TTF]TCNQ was shown to be a narrow band gap semiconductor.[6] X-ray diffraction studies of [TTF][TCNQ] revealed stacks of partially oxidized TTF molecules adjacent to anionic stacks of TCNQ molecules. This "segregated stack" motif was unexpected and is responsible for the distinctive electrical properties, i.e. high and anisotropic electrical conductivity. Since these early discoveries, numerous analogues of TTF have been prepared. Well studied analogues include tetramethyltetrathiafulvalene (Me4TTF), tetramethylselenafulvalenes (TMTSFs), and bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF, CAS [66946-48-3]).[7] Several tetramethyltetrathiafulvalene salts (called Fabre salts) are of some relevance as organic superconductors.
See also
References
- ↑ "Tetrathiafulvalene" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/99451#section=Safety-and-Hazards.
- ↑ 2.0 2.1 Bendikov, M; Wudl, F; Perepichka, D F (2004). "Tetrathiafulvalenes, Oligoacenenes, and Their Buckminsterfullerene Derivatives: The Brick and Mortar of Organic Electronics". Chemical Reviews 104 (11): 4891–4945. doi:10.1021/cr030666m. PMID 15535637.
- ↑ Wudl, F.; Kaplan, M. L. (1979). "2,2′‐Bi‐L,3‐Dithiolylidene (Tetrathiafulvalene, TTF) and its Radical Cation Salts". Inorganic Syntheses. 19. 27–30. doi:10.1002/9780470132500.ch7. ISBN 978-0-470-13250-0.
- ↑ D. Chasseau; G. Comberton; J. Gaultier; C. Hauw (1978). "Réexamen de la structure du complexe hexaméthylène-tétrathiafulvalène-tétracyanoquinodiméthane". Acta Crystallographica Section B 34 (2): 689. doi:10.1107/S0567740878003830.
- ↑ Wudl, F.; Wobschall, D.; Hufnagel, E. J. (1972). "Electrical Conductivity by the Bis(1,3-dithiole)-bis(1,3-dithiolium) System". J. Am. Chem. Soc. 94 (2): 670–672. doi:10.1021/ja00757a079.
- ↑ Ferraris, J.; Cowan, D. O.; Walatka, V. V., Jr.; Perlstein, J. H. (1973). "Electron transfer in a new highly conducting donor-acceptor complex". J. Am. Chem. Soc. 95 (3): 948–949. doi:10.1021/ja00784a066.
- ↑ Larsen, J.; Lenoir, C. (1998). "2,2'-Bi-5,6-Dihydro-1,3-Dithiolo[4,5-b][1,4]dithiinylidene (BEDT-TTF)". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv9p0072.; Collective Volume, 9, pp. 72
Further reading
- Rovira, C. (2004). "Bis(ethylenethio)tetrathiafulvalene (BET-TTF) and Related Dissymmetrical Electron Donors: From the Molecule to Functional Molecular Materials and Devices (OFETs)". Chemical Reviews 104 (11): 5289–5317. doi:10.1021/cr030663+. PMID 15535651.
- Iyoda, M; Hasegawa, M; Miyake, Y (2004). "Bi-TTF, Bis-TTF, and Related TTF Oligomers". Chemical Reviews 104 (11): 5085–5113. doi:10.1021/cr030651o. PMID 15535643.
- Frere, P.; Skabara, P. J. (2005). "Salts of Extended Tetrathiafulvalene analogues: relationships Between Molecular Structure, Electrochemical Properties and Solid State Organization". Chemical Society Reviews 34 (1): 69–98. doi:10.1039/b316392j. PMID 15643491.
- Gorgues, Alain; Hudhomme, Pietrick; Salle, Marc. (2004). "Highly Functionalized Tetrathiafulvalenes: Riding along the Synthetic Trail from Electrophilic Alkynes". Chemical Reviews 104 (11): 5151–5184. doi:10.1021/cr0306485. PMID 15535646.
- Physical properties of Tetrathiafulvalene from the literature.
- Segura, José L.; Martín, Nazario (2001). "New Concepts in Tetrathiafulvalene Chemistry". Angewandte Chemie International Edition 40 (8): 1372–1409. doi:10.1002/1521-3773(20010417)40:8<1372::aid-anie1372>3.0.co;2-i. PMID 11317287.
 |

