Chemistry:Tetramethylammonium fluoride
From HandWiki
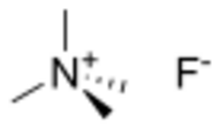
| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N,N-Trimethylmethanaminium fluoride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C4H12FN | |
| Molar mass | 93.145 g·mol−1 |
| Appearance | white solid |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H312, H315, H319, H332, H335 | |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P305+351+338, P312, P321, P322, P330, P332+313, P337+313, P362, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetramethylammonium fluoride is the quaternary ammonium salt with the formula (CH3)4NF. This hygroscopic white solid is a source of “naked fluoride": fluoride ions not complexed with a metal atom. Most other soluble salts of fluoride are in fact bifluorides, HF2–. Historically, there have been two main approaches to prepare tetramethylammonium fluoride: hydrofluoric acid neutralization of tetramethylammonium hydroxide, and salt metathesis between different ammonium salts and inorganic fluoride sources, such as KF or CsF.[1] Because the fluoride anion is extremely basic, the salt slowly reacts with acetonitrile, inducing dimerization to CH3C(NH2)=CHCN, which co-crystallizes.[2]
Related salts
- Tetramethylphosphonium fluoride (CH3)4PF forms stable acetonitrile solutions. It is prepared from the ylide and potassium bifluoride:
- (CH3)3P=CH2 + KHF2 → (CH3)4PF + KF
- Gaseous tetramethylphosphonium fluoride exists as the phosphorane but autoionizes in acetonitrile solution.[3] A more elaborate phosphazenium salt ([(CH3)2N)3P]2N+F−) is also known.[4]
- Anhydrous Tetrabutylammonium fluoride has been prepared by the reaction of hexafluorobenzene and tetrabutylammonium cyanide.[5]
References
- ↑ Iashin, Vladimir; Wirtanen, Tom; Perea-Buceta, Jesus E. (2022-02-18). "Tetramethylammonium Fluoride: Fundamental Properties and Applications in C-F Bond-Forming Reactions and as a Base" (in en). Catalysts 12 (2): 233. doi:10.3390/catal12020233. ISSN 2073-4344.
- ↑ Christe, K. O.; Wilson, W. W.; Wilson, R. D.; Bau, R.; Feng, J. A. (1990). "Syntheses, Properties, and Structures of Anhydrous Tetramethylammonium Fluoride and Its 1:1 Adduct with trans-3-Amino-2-butenenitrile". Journal of the American Chemical Society 112 (21): 7619–7625. doi:10.1021/ja00177a025.
- ↑ Kornath, Andreas; Neumann, F.; Oberhammer, H. (2003). "Tetramethylphosphonium Fluoride: "Naked" Fluoride and Phosphorane". Inorganic Chemistry 42 (9): 2894–2901. doi:10.1021/ic020663c. PMID 12716181.
- ↑ Schwesinger, Reinhard (2001). "1,1,1,3,3,3-Hexakis(dimethylamino)-1λ5,3λ5-diphosphazenium Fluoride". e-EROS Encyclopedia of Reagents for Organic Synthesis. pp. 1–2. doi:10.1002/047084289X.rh014m. ISBN 0471936235.
- ↑ Haoran Sun; Stephen G. DiMagno (2005). "Anhydrous Tetrabutylammonium Fluoride". Journal of the American Chemical Society 127 (7): 2050–1. doi:10.1021/ja0440497. PMID 15713075.
 |

