Chemistry:Tetrahydrothiophene
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Thiolane | |||
| Other names
Tetrahydrothiophene,
thiophane, tetramethylene sulfide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | THT | ||
| 102392 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2412 | ||
| |||
| |||
| Properties | |||
| C4H8S | |||
| Molar mass | 88.17 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Density | 0.997 g/mL[1] | ||
| Melting point | −96 °C (−141 °F; 177 K) | ||
| Boiling point | 119 °C (246 °F; 392 K) | ||
| Hazards | |||
| Main hazards | Stench, flammable, irritant | ||
| Safety data sheet | Oakwood | ||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| H225, H302, H312, H315, H319, H332, H412 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P273, P280, P301+312, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P312, P321, P322, P330, P332+313, P337+313, P362 | |||
| Flash point | 12 °C (54 °F; 285 K) | ||
| 200 °C (392 °F; 473 K) | |||
| Related compounds | |||
Related compounds
|
Tetrahydrofuran, Thiophene, Selenolane, Thiazolidine, Dithiolane, Thiane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
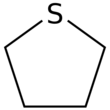
Tetrahydrothiophene is an organosulfur compound with the formula (CH2)4S. The molecule consists of a five-membered saturated ring with four methylene groups and a sulfur atom. It is the saturated analog of thiophene. It is a volatile, colorless liquid with an intensely unpleasant odor. It is also known as thiophane, thiolane, or THT.
Synthesis and reactions
Tetrahydrothiophene is prepared by the reaction of tetrahydrofuran with hydrogen sulfide. This vapor-phase reaction is catalyzed by alumina and other heterogenous acid catalysts.[2][3]
This compound is a ligand in coordination chemistry, an example being the complex chloro(tetrahydrothiophene)gold(I).[4]
Oxidation of THT gives the sulfone sulfolane, which is of interest as a polar, odorless solvent:
- C
4H
8S + 2 O → C
4H
8SO
2
Sulfolane is, however, more conventionally prepared from butadiene.
Natural occurrence
Both unsubstituted and substituted tetrahydrothiophenes are reported to occur in nature. For example, tetrahydrothiophene occurs as a volatile from Eruca sativa Mill. (salad rocket)[5] while monocyclic substituted tetrahydrothiophenes have been isolated from Allium fistulosum 'Kujou',[6] Allium sativum (garlic),[7] Allium cepa (onion),[8] Allium schoenoprasum (chives),[9] and Salacia prinoides.[10] Albomycins are a group of tetrahydrothiophene-ring containing antibiotics from streptomyces while biotin and neothiobinupharidine (and other nuphar alkaloids [11]), are examples of bicyclic and polycyclic tetrahydrothiophene-ring containing natural products, respectively.
Applications
Because of its smell, tetrahydrothiophene has been used as an odorant in LPG,[3] albeit no longer in North America. It is also used as an odorant for natural gas, usually in mixtures containing tert-butylthiol.
Tetrahydrothiophene is a Lewis base classified as a soft base and its donor properties are discussed in the ECW model.
See also
References
- ↑ "Purification of Organic Chemicals". Purification of Laboratory Chemicals. 2003. pp. 361. doi:10.1016/B978-075067571-0/50008-9. ISBN 9780750675710.
- ↑ Loev, B; Massengale, JT U. S. Patent 2,899,444, "Synthesis of Tetrahydrothiophene", 8/11/1959
- ↑ 3.0 3.1 Jonathan Swanston (2006). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_793.pub2.
- ↑ "(Tetrahydrothiophene)Gold(I) or Gold(III) Complexes". Inorganic Syntheses. 2007. pp. 85–91. doi:10.1002/9780470132579.ch17. ISBN 9780470132579.
- ↑ Aissani, N (2006). "Nematicidal Activity of the Volatilome of Eruca sativa on Meloidogyne incognita". Journal of Agricultural and Food Chemistry 63 (27): 6120–6125. doi:10.1021/acs.jafc.5b02425. PMID 26082278.
- ↑ Fukaya, M (2018). "Rare Sulfur-Containing Compounds, Kujounins A1 and A2 and Allium Sulfoxide A1, from Allium fistulosum 'Kujou'". Organic Letters 20 (1): 28–31. doi:10.1021/acs.orglett.7b03234. PMID 29227665.
- ↑ Block, E (2018). "Ajothiolanes: 3,4-Dimethylthiolane Natural Products from Garlic (Allium sativum).". Journal of Agricultural and Food Chemistry 66 (39): 10193–10204. doi:10.1021/acs.jafc.8b03638. PMID 30196701. https://www.osti.gov/servlets/purl/1490686.
- ↑ Aoyagi, M (2011). "Structure and Bioactivity of Thiosulfinates Resulting from Suppression of Lachrymatory Factor Synthase in Onion". Journal of Agricultural and Food Chemistry 59 (20): 10893–10900. doi:10.1021/jf202446q. PMID 21905712.
- ↑ Fukaya, M (2019). "Cyclic Sulfur Metabolites from Allium schoenoprasum var. foliosum". Phytochemistry Letters 29: 125–128. doi:10.1016/j.phytol.2018.11.018. Bibcode: 2019PChL...29..125F.
- ↑ Tanabe, G (2008). "Synthesis and Elucidation of Absolute Stereochemistry of Salaprinol, another Thiosugar Sulfonium Sulfate from the Ayurvedic Traditional Medicine Salacia prinoides". Tetrahedron 64 (43): 10080–10086. doi:10.1016/j.tet.2008.08.010.
- ↑ Korotkov, A (2015). "Total Syntheses and Biological Evaluation of Both Enantiomers of Several Hydroxylated Dimeric Nuphar Alkaloids". Angewandte Chemie International Edition 54 (36): 10604–10607. doi:10.1002/anie.201503934. PMID 26205039.
 |