Chemistry:Tantalum(IV) iodide
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| TaI4 | |
| Molar mass | 688.57 |
| Appearance | black solid[1] |
| Melting point | 398 °C (671 K) (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tantalum(IV) iodide is an inorganic compound with the chemical formula TaI4. It dissolves in water to give a green solution, but the color fades when left in the air and produces a white precipitate.[2]
Preparation
Tantalum(IV) iodide can be prepared by the reduction reaction of tantalum(V) iodide and tantalum.[2] If pyridine is used as the reducing agent, there is an adduct TaI4(py)2.[3]
Tantalum(IV) iodide can also be obtained by reacting tantalum(V) iodide with aluminum, magnesium or calcium at 380 °C. Ta6I14 is also formed. This makes it difficult to produce a very pure crystallized tantalum(IV) iodide.[4]
- 3 TaI
5 + Al → 3 TaI
4 + AlI
3
Properties
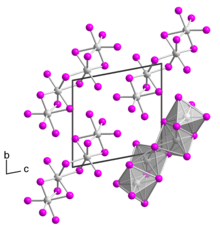
Tantalum(IV) iodide is a black solid. It has a crystal structure isotypic to that of niobium(IV) iodide.[4] Single-crystalline tantalum(IV) iodide was first obtained in 2008 by Rafal Wiglusz and Gerd Meyer as a chance product of a reaction in a tantalum ampoule that was supposed to lead to the product Rb(Pr6C2)I12.[5] The single crystal has a triclinic crystal structure with space group P1 (space group no. 2) with two formula units per unit cell (a = 707.36 pm, b = 1064.64 pm, c = 1074.99 pm, α = 100.440°, β = 89.824° and γ = 104.392°). The crystal structure differs from that of other transition metal tetraiodides, which usually have a MI4/2I2/1 chain structure, as it consists of TaI6 octahedra bridged over a common surface to form a dimer. Two such dimers bridge over a common edge to form a tetramer.[6]
References
- ↑ Georg Brauer: Handbuch der präparativen anorganischen Chemie. 3., umgearb. Auflage. Band III. Enke, Stuttgart 1981, ISBN 3-432-87823-0, pp. 1455.
- ↑ 2.0 2.1 Robert F. Rolsten (Jun 1958). "Preparation and X-ray Study of Some Tantalum Halides" (in en). Journal of the American Chemical Society 80 (12): 2952–2953. doi:10.1021/ja01545a011. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja01545a011. Retrieved 2021-03-24.
- ↑ R. E. McCarley, J. C. Boatman (Jun 1963). "The Preparation of Tantalum(IV) Bromide, Tantalum(IV) Iodide, and Pyridine Adducts of the Tantalum(IV) Halides" (in en). Inorganic Chemistry 2 (3): 547–551. doi:10.1021/ic50007a030. ISSN 0020-1669. https://pubs.acs.org/doi/abs/10.1021/ic50007a030. Retrieved 2021-03-24.
- ↑ 4.0 4.1 Handbuch der präparativen anorganischen Chemie. 3 (3., umgearb. Aufl ed.). Stuttgart: Enke. 1981. ISBN 978-3-432-87823-2.
- ↑ Meyer, Gerd; Wiglusz, Rafal; Pantenburg, Ingo; Mudring, Anja-Verena (May 2008). "Tantalum(IV) Iodide, TaI4: A Molecular Solid Consisting of Dimers of Dimers, Ta4I16" (in de). Zeitschrift für anorganische und allgemeine Chemie 634 (5): 825–828. doi:10.1002/zaac.200700529. https://onlinelibrary.wiley.com/doi/10.1002/zaac.200700529.
- ↑ Habermehl, Katja (2010). Neue Untersuchungen an Halogeniden des Niobs und Tantals (text.thesis.doctoral thesis). Universität zu Köln.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

