Chemistry:Sodium methylsulfinylmethylide
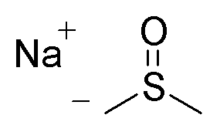
| |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium (methanesulfinyl)methanide | |
| Other names
sodium dimsylate, dimsylsodium, NaDMSYL
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | NaDMSO |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C2H5NaOS | |
| Molar mass | 100.13 |
| Appearance | White solid, solution in DMSO is green |
| Reacts forming DMSO | |
| Solubility | Very soluble in DMSO and many polar organic solvents |
| Hazards | |
| Main hazards | decomposes to corrosive NaOH, May be explosive in certain circumstances [1] |
| Related compounds | |
Related compounds
|
Dimethyloxosulfonium methylide, dimethyl sulfoxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium methylsulfinylmethylide (also called NaDMSO or dimsyl sodium) is the sodium salt of the conjugate base of dimethyl sulfoxide. This unusual salt has some uses in organic chemistry as a base and nucleophile.
Since the first publication in 1965 by Corey et al.,[2] a number of additional uses for this reagent have been identified.[3]
Preparation
Sodium methylsulfinylmethylide is prepared by heating sodium hydride[4] or sodium amide[5] in DMSO[6]
- CH3SOCH3 + NaH → CH3SOCH2−Na+ + H2
- CH3SOCH3 + NaNH2 → CH3SOCH2−Na+ + NH3
Reactions
As a Base
The pKa of DMSO is 35, which leads NaDMSO to be a powerful Brønsted base. NaDMSO is used in the generation of phosphorus and sulfur ylides.[7] NaDMSO in DMSO is especially convenient in the generation of dimethyloxosulfonium methylide and dimethylsulfonium methylide.[2][8]
Reaction with esters
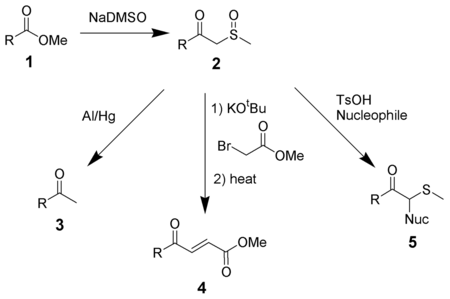
NaDMSO condenses with esters (1) to form β-ketosulfoxides (2), which can be useful intermediates.[9] Reduction of β-ketosulfoxides with aluminium amalgam gives methyl ketones (3).[10] Reaction with alkyl halides followed by elimination gives α,β-unsaturated ketones (4). β-ketosulfoxides can also be used in the Pummerer rearrangement to introduce nucleophiles alpha to a carbonyl (5).[11]
References
- ↑ "Sodium Hydride in Aprotic Solvents: Look Out". https://www.science.org/content/blog-post/sodium-hydride-aprotic-solvents-look-out.
- ↑ 2.0 2.1 Corey, E. J.; Chaykovsky, M. (1965). "Methylsulfinyl Carbanion (CH3-SO-CH2−). Formation and Applications to Organic Synthesis". J. Am. Chem. Soc. 87 (6): 1345–1353. doi:10.1021/ja01084a033.
- ↑ Mukulesh Mondal "Sodium methylsulfinylmethylide: A versatile reagent" Synlett 2005, vol. 17, 2697-2698. doi:10.1055/s-2005-917075
- ↑ Iwai, I.; Ide, J. (1988). "2,3-Diphenyl-1,3-Butadiene". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv6p0531.; Collective Volume, 6, pp. 531
- ↑ Kaiser, E. M.; Beard, R. D.; Hauser, C. R. (1973). "Preparation and reactions of the mono- and dialkali salts of dimethyl sulfone, dimethyl sulfoxide, and related compounds". J. Organomet. Chem. 59: 53–64. doi:10.1016/S0022-328X(00)95020-4.
- ↑ "Preparation of dimsyl sodium". http://stenutz.eu/sop/sop401.html.
- ↑ Romo, D.; Myers, A. I. (1992). "An asymmetric route to enantiomerically pure 1,2,3-trisubstituted cyclopropanes". J. Org. Chem. 57 (23): 6265–6270. doi:10.1021/jo00049a038.
- ↑ Trost, B. M.; Melvin, L. S., Jr. (1975). Sulfur Ylides: Emerging Synthetic Intermediates. New York: Academic Press. ISBN 0-12-701060-2.
- ↑ Ibarra, C. A; Rodgríguez, R. C; Monreal, M. C. F; Navarro, F. J. G.; Tesoreo, J. M. (1989). "One-pot synthesis of β-keto sulfones and β-keto sulfoxides from carboxylic acids". J. Org. Chem. 54 (23): 5620–5623. doi:10.1021/jo00284a043.
- ↑ Swenton, J. S.; Anderson, D. K.; Jackson, D. K.; Narasimhan, L. (1981). "1,4-Dipole-metalated quinone strategy to (±)-4-demethoxydaunomycinone and (±)-daunomycinone. Annelation of benzocyclobutenedione monoketals with lithioquinone bisketals". J. Org. Chem. 46 (24): 4825–4836. doi:10.1021/jo00337a002.
- ↑ Isibashi, H.; Okada, M.; Komatsu, H.; Ikeda, M. S. (1985). "A New Synthesis of Substituted Cyclopentenones by Olefin Cyclization Initiated by Pummerer Reaction Intermediates". Synthesis 1985 (6/7): 643–645. doi:10.1055/s-1985-31290.
External links
- "The Dimethyl Sulfoxide (DMSO) Anion — Dimsyl Ion". Gaylord Chemical Corporation. October 2007. http://www.gaylordchemical.com/bulletins/bulletin110b/Bulletin110B.pdf.
- "Preparation of dimsyl sodium". June 2009. http://stenutz.eu/sop/sop401.html.
 |