Chemistry:Seleninic acid
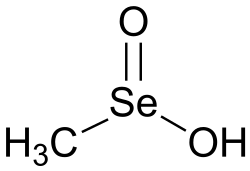
A seleninic acid is an organoselenium compound and an oxoacid with the general formula RSeO
2H, where R ≠ H. Its structure is R–Se(=O)–OH. It is a member of the family of organoselenium oxoacids, which also includes selenenic acids and selenonic acids, which are R–Se–OH and R–Se(=O)
2–OH, respectively. The parent member of this family of compounds is methaneseleninic acid (CH
3–Se(=O)–OH), also known as methylseleninic acid or "MSA".
Reactions and applications in synthesis
Seleninic acids (particularly areneseleninic acids) are useful catalysts for hydrogen peroxide epoxidations, Baeyer–Villiger oxidations, oxidations of thioethers, etc.; peroxyseleninic acids (R–Se(=O)–OOH) are thought to be the active oxidants.[1][2][3]
Structure, bonding, properties
Methaneseleninic acid has been characterized by X-ray crystallography.[4] The configuration about the selenium atom is pyramidal, with Se-C = 1.925(8) Å, Se-O = 1.672(7) Å, Se-OH = 1.756(7) Å, the angle OSeO = 103.0(3)°, the angle HO-Se-C = 93.5(3)°, and the angle OSeC = 101.4(3)°. The structure is isomorphous to that of methanesulfinic acid [5]
Benzeneseleninic acid (C
6H
5–Se(=O)–OH) had been previously characterized by X-ray methods[6] and its optical resolution reported.[7]
References
- ↑ ten Brink, G.-J.; Fernandes, B. C. M.; van Vliet, M. C. A.; Arends, I. W. C. E.; Sheldon, R. A. "Selenium catalyzed oxidations with aqueous hydrogen peroxide. Part I. Epoxidation reactions in homogeneous solution." J. Chem. Soc., Perkin Trans., 1 2001, 224–228. doi: 10.1039/b008198l
- ↑ ten Brink, G.-J.; Vis, J.-M.; Arends, I. W. C. E.; Sheldon, R. A. "Selenium-Catalyzed Oxidations with Aqueous Hydrogen Peroxide. 2. Baeyer-Villiger Reactions in Homogeneous Solution." J. Org. Chem. 2001, 66, 2429–2433. doi: 10.1021/jo0057710
- ↑ Mercier, E. A.; Smith, C. D.; Parvez, M.; Back, T. G. "Cyclic Seleninate Esters as Catalysts for the Oxidation of Sulfides to Sulfoxides, Epoxidation of Alkenes, and Conversion of Enamines to α-Hydroxyketones." J. Org. Chem. 2012, 77, 3508–3517. doi: 10.1021/jo300313v
- ↑ Block, E.; Birringer, M.; Jiang, W.; Nakahodo, T.; Thompson, H. J.; Toscano, P. J.; Uzar, H.; Zhang, X.; Zhu, Z. "Allium chemistry: Synthesis, natural occurrence, biological activity, and chemistry of Se-alk(en)ylselenocysteines and their γ-glutamyl derivatives and oxidation products." J. Agric. Food Chem. 2001, 49, 458–470. doi: 10.1021/jf001097b
- ↑ Seff, K.; Heidner, E. G.; Meyers, M.; Trueblood, K. N. "The crystal and molecular structure of methanesulfinic acid." Acta Crystallographica Section B 1969, 25, 350–354.
- ↑ J. H. Bryden, H.; McCullough, J. D. "The crystal structure of benzeneseleninic acid." Acta Crystallogr. 1954. 7, 833–838. doi:10.1107/S0365110X54002551
- ↑ Toshio, S.; Watanabe, I.; Kamigata, N. "Optically active seleninic acids: optical resolution and stability." Angew. Chem., Int. Edn. 2001, 40, 2460–2462. doi: 10.1002/1521-3773(20010702)40:13<2460::AID-ANIE2460>3.0.CO;2-Q
 |