Chemistry:Scandium perrhenate
| |
| Names | |
|---|---|
| IUPAC name
Scandium perrhenate(VI)
| |
| Other names
Scandium(III) perrhenate
Scandium(III) perrhenate(VI) | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| |
| |
| Properties | |
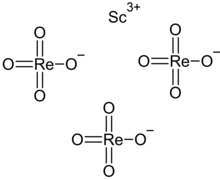
| Sc(ReO4)3 | |
| Molar mass | 795.577 (anhydrous) 813.593 (monohydrate) 849.625 (trihydrate) |
| Melting point | 735°C[1] |
| very soluble | |
| Related compounds | |
Other anions
|
Scandium nitrate Scandium perchlorate |
Other cations
|
Yttrium perrhenate Lanthnaum perrhenate |
Related compounds
|
Rhenium(VII) oxide Perrhenic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Scandium perrhenate is an inorganic compound, with the chemical formula Sc(ReO4)3. Its thermal stability is lower than that of the corresponding compounds of the yttrium and lanthanum perrhenates.[2][3]
Preparation and properties
Scandium perrhenate can be obtained by reacting perrhenic acid with scandium oxide.[1] From the solution, the trihydrate of scandium perrhenate can be precipitated, which loses water at 50 °C to obtain Sc(ReO4)3·H2O, and obtains the anhydrous form at 140 °C. Scandium oxide and rhenium(VII) oxide are formed at 550 °C.[4]
Scandium perrhenate trihydrate is a crystal in the triclinic crystal system, with space group P1, a=7.333, b=7.985, c=20.825 Å; α=93.35, β=92.20, γ=97.42°.[5]
Scandium perrhenate can crystallize with ammonium perrhenate in water to form NH4Sc(ReO4)4·4H2O.[6]
References
- ↑ 1.0 1.1 Yi, Xianwu; et al. Series of Inorganic Chemistry - Vol 7. Scandium; Rare Earth Elements. Science Press. pp. 57. 3.9.6 Manganates and Rhenates of Scandium. (in Chinese)
- ↑ Ovchinnikov, K. V.; Nikolaev, E. N.; Semenov, G. A. Mass-spectrometric study of the in vacuo thermal decomposition of scandium, yttrium, and lanthanum perrhenates(in Russian). Zhurnal Obshchei Khimii, 1983. 53 (5): 966-968. ISSN: 0044-460X.
- ↑ "ВАЛЕНТИНА МИХАЙЛОВНА БЕРЕСТОВИЦКАЯ (1940-2017)". Журнал Общей Химии 90 (8): 1151–1152. 2020-08-01. doi:10.31857/s0044460x20080016. ISSN 0044-460X. http://dx.doi.org/10.31857/s0044460x20080016.
- ↑ Komissarova, L. N.; Varfolomeev, M. B.; Ivanov, V. I.; Plyushchev, V. E. Preparation and properties of scandium perrhenates(in Russian). Doklady Akademii Nauk SSSR, 1965. 160 (3): 608-611. ISSN 0002-3264.
- ↑ Khrustalev, V. N.; Varkholomeev, M. B.; Struchkov, Yu. T. Crystal structure of scandium perrhenate trihydrate(in Russian). Zhurnal Neorganicheskoi Khimii, 1997. 42 (11): 1779-1784. ISSN 0044-457X.
- ↑ Khrustalev, V. N.; Varfolomeev, M. B.; Shamrai, N. B.; Struchkov, Yu. T. Synthesis and crystal structure of double ammonium indium and ammonium scandium perrhenate tetrahydrates(in Russian). Zhurnal Neorganicheskoi Khimii, 1996. 41 (4): 549-553. ISSN 0044-457X.
 |

