Chemistry:Sabinene

| |
| Names | |
|---|---|
| IUPAC name
4-methylene-1-(1-methylethyl)bicyclo[3.1.0]hexane
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C10H16 | |
| Molar mass | 136.23 g/mol |
| Density | 0.844 g/mL at 20 °C g/cm3 |
| Boiling point | 163 to 164 °C (325 to 327 °F; 436 to 437 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sabinene is a natural bicyclic monoterpene with the molecular formula C10H16. It is isolated from the essential oils of a variety of plants including Marjoram,[2] holm oak (Quercus ilex) and Norway spruce (Picea abies). It has a strained ring system with a cyclopentane ring fused to a cyclopropane ring.
Sabinene is one of the chemical compounds that contributes to the spiciness of black pepper and is a major constituent of carrot seed oil. It also occurs in tea tree oil at a low concentration. It is also present in the essential oil obtained from nutmeg,[3] Laurus nobilis, and Clausena anisata.
Biosynthesis
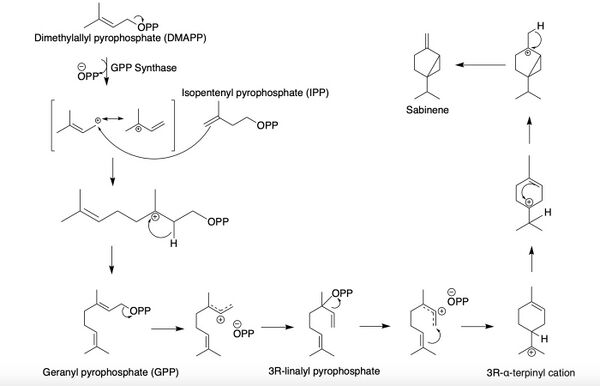
Sabinene, a bicyclic monoterpene, is present in the (+) and (-) enantiomers.[4] It is biosynthesized from the common terpenoid precursor, geranyl pyrophosphate (GPP) that undergoes polycyclization catalyzed by sabinene synthase (SabS).[4][5] GPP is formed from the terpenoid synthesis pathway with the starter units, isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). The starter units, IPP and DMAPP, can be synthesized from either the mevalonate (MVA) or the methylerythritol 4-phosphate (MEP) pathway.[4] With the head-to-tail condensation of IPP and DMAPP catalyzed by GPP synthase, GPP is formed. Sabinene synthase (SabS) then catalyzes the ionization and isomerization of GPP to form 3R-linalyl pyrophosphate.[4][6] Further ionization and cyclization results in the formation of sabinene.
See also
- Thujene, a double bond isomer of sabinene
References
- ↑ Beilstein. 5, IV, 451
- ↑ Verma, Ram S.; Padalia, Rajendra C.; Chauhan, Amit; Verma, Rajesh K.; Ur Rahman, Laiq; Singh, Anand (2016). "Changes in the Essential Oil Composition of Origanum majoranaL. During Post Harvest Drying". Journal of Essential Oil Bearing Plants 19 (6): 1547–1552. doi:10.1080/0972060X.2014.935039. http://www.tandfonline.com/doi/abs/10.1080/0972060X.2014.935039.
- ↑ Shulgin, A. T.; Sargent, T.; Naranjo, C. (1967). "The Chemistry and Psychopharmacology of Nutmeg and of Several Related Phenylisopropylamines" (pdf). Psychopharmacology Bulletin 4 (3): 13. PMID 5615546. http://bitnest.ca/external.php?id=%250E%253D9%250F%2524G%252F%2518B%255B%255B4%2522.FXQ%255CO%2500TK.
- ↑ 4.0 4.1 4.2 4.3 4.4 Cao, Yujin; Zhang, Haibo; Liu, Hui; Liu, Wei; Zhang, Rubing; Xian, Mo; Liu, Huizhou (2018-02-01). "Biosynthesis and production of sabinene: current state and perspectives" (in en). Applied Microbiology and Biotechnology 102 (4): 1535–1544. doi:10.1007/s00253-017-8695-5. ISSN 1432-0614. PMID 29264773. https://doi.org/10.1007/s00253-017-8695-5.
- ↑ Peters, R.J.; Croteau, R. B. (2003-09-15). "Alternative termination chemistries utilized by monoterpene cyclases: chimeric analysis of bornyl diphosphate, 1,8-cineole, and sabinene synthases" (in en). Archives of Biochemistry and Biophysics 417 (2): 203–211. doi:10.1016/S0003-9861(03)00347-3. ISSN 0003-9861. PMID 12941302. https://www.sciencedirect.com/science/article/abs/pii/S0003986103003473.
- ↑ 6.0 6.1 Adam, K. P.; Croteau, R. (1998-09-28). "Monoterpene Biosynthesis in the Liverwort Conocephalum Conicum: Demonstration of Sabinene Synthase and Bornyl Diphosphate Synthase in Honour of Professor G. H. Neil Towers 75th Birthday" (in en). Phytochemistry 49 (2): 475–480. doi:10.1016/S0031-9422(97)00741-3. ISSN 0031-9422. PMID 9747540. Bibcode: 1998PChem..49..475A. https://www.sciencedirect.com/science/article/abs/pii/S0031942297007413.
 |