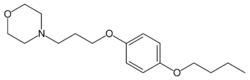
Chemistry:Pramocaine
 | |
| Clinical data | |
|---|---|
| Trade names | Analpram HC, Caladryl, Caladryl Clear, Cortane-B, Epifoam, Gold Bond Maximum Relief, Itch-X, Pramosone, Prax, Proctodan-HC, Proctofoam, Tronolane, Vagisil Medicated |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a682429 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Topical, rectal, Vaginal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H27NO3 |
| Molar mass | 293.407 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Pramocaine (INN and BAN, also known as pramoxine or pramoxine HCl) is a topical anesthetic discovered at Abbott Laboratories in 1953[1] and used as an antipruritic. During research and development, pramocaine hydrochloride stood out among a series of alkoxy aryl alkamine ethers as an especially good topical local anesthetic agent.[1] Pharmacologic study revealed it to be potent and of low acute and subacute toxicity, well tolerated by most mucous membranes and of a low sensitizing index in humans.[1] Like other local anesthetics, pramocaine decreases the permeability of neuronal membranes to sodium ions, blocking both initiation and conduction of nerve impulses. Depolarization and repolarization of excitable neural membranes is thus inhibited, leading to numbness.
Use
Topical anesthetics are used to relieve pain and itching caused by conditions such as sunburn or other minor burns, insect bites or stings, poison ivy, poison oak, poison sumac, and minor cuts and scratches.[2] The popular itch creams Gold Bond and some forms of calamine lotion use pramocaine hydrochloride to numb sensitive skin, as does the pain relief variant of Neosporin and some formulations of Sarna. The hydrochloride salt form of pramocaine is water-soluble.
Pramocaine is a common component of over the counter hemorrhoid preparations.
Pramocaine is also included in some topical antibiotics like Neosporin Plus Pain Relief used to treat or prevent infections due to its pain releving effects. However, there is no additional antibiotic effect compared to antibiotics without pramocaine.[3]
Synthesis
The ether formation between hydroquinone (1) and 1-bromobutane (2) gives 4-butoxyphenol [122-94-1] (3). Alkylation with 4-(3-chloropropyl)morpholine [57616-74-7] (4) gives pramocaine (5).
See also
References
- ↑ 1.0 1.1 1.2 "The Pharmacology of Pramoxine Hydrochloride: A New Topical Local Anesthetic". Current Researches in Anesthesia & Analgesia 32 (6:1): 418–25. 1953. PMID 13107298.
- ↑ "Pramoxine". MedlinePlus Drug Information. U.S. National Library of Medicine. September 25, 2013. https://www.nlm.nih.gov/medlineplus/druginfo/meds/a682429.html.
- ↑ "Neosporin Plus Pain Relief Ointment, TriBiozene Ointment (neomycin/polymyxin B/ bacitracin/pramoxine) dosing, indications, interactions, adverse effects, and more". https://reference.medscape.com/drug/neosporin-plus-pain-relief-ointment-tribiozene-ointment-neomycin-polymyxin-b-bacitracin-pramoxine-999349.
- ↑ Salva Salvatore Joseph De & Migliarese Joseph Francis, U.S. Patent 3,172,805 (1965 to Colgate Palmolive Co).
- ↑ Li Yuedong, et al. CN patent 106045942 (2016 to Shandong Chengchuang Blue Sea Pharmaceutical Technology Co., Ltd.).
 |


