Chemistry:Pinacol rearrangement
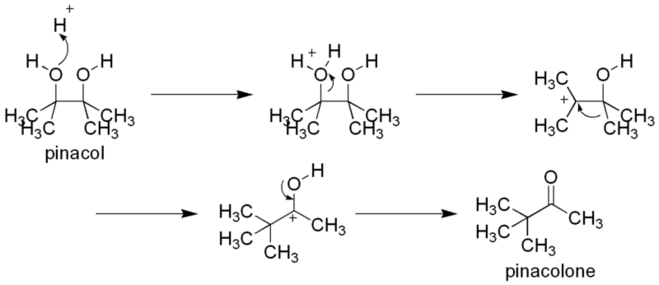
The pinacol–pinacolone rearrangement is a method for converting a 1,2-diol to a carbonyl compound in organic chemistry. The 1,2-rearrangement takes place under acidic conditions. The name of the rearrangement reaction comes from the rearrangement of pinacol to pinacolone.[1]
This reaction was first described by Wilhelm Rudolph Fittig (of Fittig reaction fame) in 1860.[2]
Mechanism
In the course of this organic reaction, protonation of one of the –OH groups occurs and a carbocation is formed. If the –OH groups are not alike (i.e. the pinacol is asymmetrical), then the one which creates a more stable carbocation participates in the reaction. Subsequently, an alkyl group from the adjacent carbon migrates to the carbocation center.
The driving force for this rearrangement step is believed to be the relative stability of the resultant oxonium ion. Although the initial carbocation is already tertiary, the oxygen can stabilize the positive charge much more favorably due to the complete octet configuration at all centers. It can also be seen as the -OH's lone pairs pushing an alkyl group off as seen in the asymmetrical pinacol example. The migration of alkyl groups in this reaction occurs in accordance with their usual migratory aptitude, i.e.phenyl carbocation > hydride > tertiary carbocation (if formed by migration) > secondary carbocation (if formed by migration) > methyl carbocation. {Why carbocation? Because every migratory group leaves by taking electron pair with it.} The conclusion is that the group which stabilizes the carbocation more effectively is migrated.
Example of asymmetrical pinacol rearrangement
When a pinacol is not symmetrical, there is a choice for which hydroxyl group will leave and which alkyl shift will occur. The selectivity will be determined by the stability of the carbocations. In this case although both choices are tertiary, the phenyl groups result in significantly higher stabilization of the positive charge through resonance.
Stereochemistry of the rearrangement
In cyclic systems, the reaction presents more features of interest. In these reactions, the stereochemistry of the diol plays a crucial role in deciding the major product. An alkyl group which is situated trans- to the leaving –OH group may migrate to the carbocation center, but cis- alkyl groups migrate at a very low rate. In the absence of trans- alkyl groups, ring contraction may occur as the major product instead, i.e. the ring carbon itself may migrate.[3][4] This reveals another interesting feature of the reaction, viz. that it is largely concerted. There appears to be a connection between the migration origin and migration terminus throughout the reaction.
Moreover, if the migrating alkyl group has a chiral center as its key atom, the configuration at this center is retained even after migration takes place.
History
Although Fittig first published about the pinacol rearrangement, it was not Fittig but Aleksandr Butlerov who correctly identified the reaction products involved.[5]
In an 1859 publication Wilhelm Rudolph Fittig described the reaction of acetone with potassium metal.[6] Fittig wrongly assumed a molecular formula of (C3H3O)n for acetone, the result of a long-standing atomic weight debate finally settled at the Karlsruhe Congress in 1860. He also wrongly believed acetone to be an alcohol which he hoped to prove by forming a metal alkoxide salt. The reaction product he obtained instead he called paraceton which he believed to be an acetone dimer. In his second publication in 1860 he reacted paraceton with sulfuric acid (the actual pinacol rearrangement).
Again Fittig was unable to assign a molecular structure to the reaction product which he assumed to be another isomer or a polymer. Contemporary chemists who had already adapted to the new atomic weight reality did not fare better. One of them, Charles Friedel, believed the reaction product to be the epoxide tetramethylethylene oxide[7] in analogy with reactions of ethylene glycol. Finally Butlerov in 1873 came up with the correct structures after he independently synthesised the compound trimethylacetic (pivalic) acid which Friedel had obtained earlier by oxidizing with a dichromate.[8]
Some of the problems during the determination of the structure are because carbon skeletal rearrangements were unknown at that time and therefore the new concept had to be found. Butlerov theory allowed the structure of carbon atoms in the molecule to rearrange and with this concept a structure for pinacolone could be found.
See also
- Benzilic acid rearrangement
- Semipinacol rearrangement
- Tiffeneau–Demjanov rearrangement, in which the leaving group is a diazo (from amine) rather than oxonium (from hydroxyl)
References
- ↑ Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012). Organic chemistry (2nd ed.). New York: Oxford University Press. p. 945. ISBN 978-0-19-927029-3.
- ↑ Wilhelm Rudolph Fittig (1860). "Über einige Derivate des Acetons". Annalen der Chemie und Pharmacie 114 (1): 54–63. doi:10.1002/jlac.18601140107. https://zenodo.org/record/1427149.
- ↑ De Petris, Giulia.; Giacomello, Pierluigi.; Picotti, Tito.; Pizzabiocca, Adriano.; Renzi, Gabriele.; Speranza, Maurizio. (November 1986). "Stereochemical effects in the gas-phase pinacol rearrangement of cis- and trans-1,2-dimethylcyclopentane-1,2-diol" (in en). Journal of the American Chemical Society 108 (24): 7491–7495. doi:10.1021/ja00284a009. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00284a009.
- ↑ De Petris, Giulia.; Giacomello, Pierluigi.; Pizzabiocca, Adriano.; Renzi, Gabriele.; Speranza, Maurizio. (February 1988). "Stereochemical effects in the gas-phase pinacol rearrangement. 2. Ring contraction versus methyl migration in cis- and trans-1,2-dimethylcyclohexane-1,2-diol" (in en). Journal of the American Chemical Society 110 (4): 1098–1103. doi:10.1021/ja00212a017. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00212a017.
- ↑ Jerome A. Berson (2002). "What Is a Discovery? Carbon Skeletal Rearrangements as Counter-Examples to the Rule of Minimal Structural Change". Angewandte Chemie International Edition 41 (24): 4655–60. doi:10.1002/anie.200290007. PMID 12481317.
- ↑ W. R. Fittig (1859). "Ueber einige Metamorphosen des Acetons der Essigsäure". Annalen der Chemie und Pharmacie 110 (1): 23–45. doi:10.1002/jlac.18591100104. https://zenodo.org/record/1427131.
- ↑ Charles Friedel (1869). "Recherches sur les acétones et sur les aldéhydes". Annales de chimie et de physique. Série 4 16: 310. http://gallica.bnf.fr/ark:/12148/bpt6k34826f/f309.table.
- ↑ Aleksandr Butlerov (1873). "Ueber Trimethylessigsäure". Justus Liebigs Annalen der Chemie und Pharmacie 170 (1–2): 151–162. doi:10.1002/jlac.18731700114. https://zenodo.org/record/1427327.
 |