Chemistry:Phosphonium iodide
| |
| Names | |
|---|---|
| IUPAC name
Phosphanium iodide
| |
| Other names
Iodine phosphide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| PH4I | |
| Molar mass | 161.910 g/mol |
| Boiling point | 62 °C (144 °F; 335 K) Sublimes[1] |
| decomposes | |
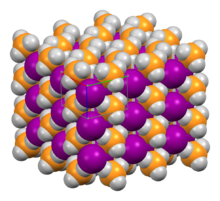
| Structure | |
| Tetragonal (P4/nmm) | |
a = 6.34 Å, c = 4.62 Å
| |
Lattice volume (V)
|
185.7 Å3 |
Formula units (Z)
|
2 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phosphonium iodide is a chemical compound with the formula PH4I. It is an example of a salt containing an unsubstituted phosphonium cation (PH+4). Phosphonium iodide is commonly used as storage for phosphine[2] and as a reagent for substituting phosphorus into organic molecules.[3]
Preparation
Phosphonium iodide is prepared by mixing diphosphorus tetraiodide (P2I4) with elemental phosphorus and water at 80 °C and allowing the salt to sublime.[4][5]
- 10 P
2I
4 + 13 P
4 + 128 H
2O → 40 PH
4I + 32 H
3PO
4
Properties
Structure
Its crystal structure has the tetragonal space group P4/nmm, which is a distorted version of the NH4Cl crystal structure; the unit cell has approximate dimensions 634×634×462 pm.[6] The hydrogen bonding in the system causes the PH+4 cations to orient such that the hydrogen atoms point toward the I− anions.[7]
Chemical
At 62 °C and atmospheric pressure, phosphonium iodide sublimates and dissociates reversibly into phosphine and hydrogen iodide (HI).[1] It oxidizes slowly in air to give iodine and phosphorus oxides; it is hygroscopic[4] and is hydrolyzed into phosphine and HI:[8]
- PH
4I ⇌ PH
3 + HI
Phosphine gas may be devolved from phosphonium iodide by mixing an aqueous solution with potassium hydroxide:[9]
- PH
4I + KOH → PH
3 + KI + H
2O
It reacts with elemental iodine and bromine in a nonpolar solution to give phosphorus halides; for example:
- 2PH
4I + 5I
2 → P
2I
4 + 8HI[4]
Phosphonium iodide is a powerful substitution reagent in organic chemistry; for example, it can convert a pyrilium into a phosphinine via substitution.[3] In 1951, Glenn Halstead Brown found that PH4I reacts with acetyl chloride to produce an unknown phosphine derivative, possibly CH
3C(=PH)PH
2 · HI.[4]
References
- ↑ 1.0 1.1 Smith, Alexander.; Calvert, Robert Peyton. (July 1914). "The Dissociation Pressures of Ammonium- and Tetramethylammonium Halides and of Phosphonium Iodide and Phosphorus Pentachloride". Journal of the American Chemical Society 36 (7): 1363–1382. doi:10.1021/ja02184a003. https://pubs.acs.org/doi/pdf/10.1021/ja02184a003. Retrieved 6 October 2020.
- ↑ Morrow, B. A.; McFarlane, Richard A. (July 1986). "Trimethylgallium adsorbed on silica and its reaction with phosphine, arsine, and hydrogen chloride: an infrared and Raman study". The Journal of Physical Chemistry 90 (14): 3192–3197. doi:10.1021/j100405a029. ISSN 0022-3654. https://pubs.acs.org/doi/pdf/10.1021/j100405a029.
- ↑ 3.0 3.1 Mei, Yanbo (2020). Complexes, Heterocycles, and Depolymerizable Polymers. Made from Building Blocks with Low-coordinated Phosphorus (Thesis). ETH Zurich. p. 18. doi:10.3929/ethz-b-000431853. hdl:20.500.11850/431853. Retrieved 6 October 2020.
- ↑ 4.0 4.1 4.2 4.3 Brown, Glenn Halstead (1951). Reactions of phosphine and phosphonium iodide (PhD). Iowa State College. Retrieved 5 Oct 2020.
- ↑ Work, J. B.; Mattern, J. A.; Antonucci, R. (5 January 2007). "Phosphonium Iodide". Inorganic Syntheses: 141–144. doi:10.1002/9780470132333.ch41.
- ↑ Dickinson, Roscoe G. (July 1922). "The Crystal Structure of Phosphonium Iodide". Journal of the American Chemical Society 44 (7): 1489–1497. doi:10.1021/ja01428a015. https://zenodo.org/record/2218537.
- ↑ Sequeira, A.; Hamilton, Walter C. (September 1967). "Hydrogen Bonding in Phosphonium Iodide: A Neutron-Diffraction Study". The Journal of Chemical Physics 47 (5): 1818–1822. doi:10.1063/1.1712171. Bibcode: 1967JChPh..47.1818S.
- ↑ Levchuk, Ievgen (2017). Design and optimization of luminescent semiconductor nanocrystals for optoelectronic applications (PDF) (faculty). University of Erlangen–Nuremberg. p. 140. Retrieved 6 Oct 2020.
- ↑ Osadchenko, Ivan M; Tomilov, Andrei P (30 June 1969). "Phosphorus Hydrides". Russian Chemical Reviews 38 (6): 495–504. doi:10.1070/RC1969v038n06ABEH001756. Bibcode: 1969RuCRv..38..495O.
 |

