Chemistry:Phosphetene
| |||
| Identifiers | |||
|---|---|---|---|
| |||
3D model (JSmol)
|
| ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
| ||
| |||
| |||
| Properties | |||
| C3H5P | |||
| Molar mass | 72.047 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
A phosphetene is an unsaturated four-membered organophosphorus heterocycle containing one phosphorus atom.[1] It is a heavier analog of an azetine, or dihydroazete. The first synthesis of a stable, isolable phosphetene was reported in 1985 by Mathey et al.[2] via ring expansion of a phosphirene-metal carbonyl complex. Since then, other synthesis routes for phosphetenes have been developed such as cyclization of phosphabutadienes, [2 + 2] cycloaddition, intramolecular arrangement, addition, and through organometallic intermediates.
The latter is of interest to the application, where synthesis through organometallic intermediates led to colored phosphetene compounds that were able to be incorporated in OLED devices.[3] Phosphetenes can also participate in reactions involving the lone pair of electrons at the phosphorus, ring opening, or ring expansion.
Nomenclature
According to the extended Hantzsch-Widman system of naming heterocyclic parent hydrides, the saturated four-membered ring containing one phosphorus atom is called a phosphetane.[4] The presence of one double bond gives a name of phosphetene. Alternately, it can be called a dihydrophosphete, as a phosphete is the cyclobutadiene-like structure containing two double bonds.
There are different constitutional isomers possible, depending on whether the double bond is attached to the phosphorus or not. The phosphorus is assigned position number 1 of the ring, and then the other structural features can be numbered relative to it. They can be distinguished based on where the double bond is in the phosphetene (Δ1-phosphetene vs Δ2-phosphetene) or where the double bond is missing as compared with the phosphete (1,2-dihydrophosphete vs 3,4-dihydrophosphete, the latter also called 2,3-dihydrophosphete).[5]
Synthesis
Via ring expansion
Isolation of the first phosphetene was reported by Mathey et al. in 1985 by the thermolysis of phosphirene-metal pentacarbonyl complexes in the presence of carbon monoxide.[2] This resulted in a 34% yield of a solid phosphorus analogue of unsaturated β-lactams with orange color, where the phosphorus atom was coordinated to the metal pentacarbonyl complex. Mathey et al. then proceeded to decomplex the metal pentacarbonyl complex from the phosphetene, but oxidation at the phosphorus takes place spontaneously, resulting in a λ5σ4 1,2-dihydrophosphete oxide solid with a yellow color.
The reaction of phosphatriafulvenes with azides resulted in the ring expansion into 1H-2-iminophosphetes as reported by Regitz et al. in 1994.[6] However, in the presence of excess azide, the Staudinger reaction can take place, which transforms the yellow λ3σ3 1H-2-iminophosphete into a λ5σ4 iminophosphorane product.
Streubel et al. (1999) were exploring the reaction of phosphirene-metal complexes with various reactants and found that a 1,2-dihydro-1-phosphet-2-one complex can be obtained, in a mixture with other compounds, from the reaction of phosphirene-metal complexes with diethylamine.[7]
Via cyclization of phosphabutadienes
Ring formation from phosphabutadienes was observed in when Nielson et al. (1987) obtained 1,2-dihydrophosphetes from the reaction of 1-[bis(trimethylsilyl)amino]phosphadiene with Me3SiN3 or elemental sulfur through a 3-coordinate (methylene)phosphorane intermediate.[8]

Grützmaker et al. (1993) reacted halogenated ylides with AlCl3 to form dihydrophosphetium salts in an intramolecular cyclization reaction.[9] Aromaticity is restored by subsequent reaction with pyridine, then a strong base, sodium bis(trimethylsilyl)amide, to form a neutral λ5-phosphete with a highly polarized P=C bond.
An air-stable, colorless, isolable 1,2-dihydrophosphete intermediate was discovered by Angelici et al. in 1993 during the synthesis of phosphaalkynes from phosphalkenes.[10]
Streubel et al. (1997) synthesized a η1-3,4-dihydrophosphete ligand complexed to metal pentacarbonyl from η1-2-phosphabutadiene complexes, which was in turn synthesized from the reaction of metal carbene complexes with a chlorophosphane.[11]
Via [2 + 2] cycloaddition reactions
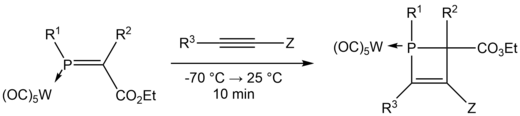
The formation of 1,2-dihydrophosphetes from [2 + 2] cycloaddition reactions involves the reaction of metal phosphaalkane complexes with alkenes or alkynes as reported by various groups, such as Mathey et al. (1990)[12] and Boese et al. (1994).[13]
Via intramolecular rearrangement
Niecke et al. (1994) reacted three equivalents of iminophosphoranes with a diyne, which resulted in a 1,2-dihydrophosphete attached to a diphosphole ring, with the formation of intermediate diphosphetene compounds.[14]
In 1995, Fluck et al. obtained 3,4-dihydrophosphetes from the reaction of 1,3-diphosphetes with CS2, COS, or CO2.[15]
Via addition reaction
The 2,4-diphosphoniodihydrophosphetide cation is an unusually stable synthetic intermediate discovered by Schmidpeter et al. in 1996 from the addition reaction of 1,3-diphosphoniopropenide with chlorophosphines.[16]
Via organometallic intermediates
The synthesis of 1,2-dihydrophosphetes by Doxsee et al. in 1989 from diphenyltitanacyclobutene (prepared from Tebbe's reagent) and dichlorophenylphosphine was remarkable due to the clean synthesis and workup and the obtained white 1,2-dihydrophosphetes were inert toward oxidation and in high yield (66%).[17] Mathey et al. (1996) also used Tebbe's reagent to prepare a novel bidentate ligand consisting of phosphorus heterocycles of 1-phosphinine-1,2-dihydrophosphetes.[18]
Majoral et al. (1997) were able to synthesize a 1,2-dihydrophosphete-zirconium complex from intramolecular coupling of dialkynyl phosphane and zirconocene-benzyne.[19]
The complex was then treated with an acid to yield a π-extended dihydrophosphetes. This method was applied by Hissler et al. in 2014 to observe the optical and redox properties of π-extended dihydrophosphetes.[3] With the introduction of various electron-rich substiuents on the π-backbone, the dihydrophosphetes displayed shifting of color in the visible region varying from blue to green, which was tested in a multilayer OLED device. As of 2023, Cummins et al. have been able to synthesize free, uncomplexed phosphet-2-ones with high yield using a phosphinidene transfer agent derived from anthracene.[20] The reactivity of the phosphet-2-ones with stabilized Wittig reagents at 100 °C led to a high yield of 1,2-dihydrophosphete products with a backbone structure that resembles Hissler's polyunsaturated dihydrophosphetes used in the OLED devices.
Reactivity
Reactivity at the phosphorus atom
Phosphetenes that have a lone pair at the phosphorus atom behave similarly to a two-electron P-donor, such as the ability to coordinate to metals. Doxsee et al. (1991) discovered that structural changes in phosphetene metal complexes are consistent with an increased s-character of the phosphorus.[21]
Ring-opening followed by cycloaddition
Ring-opening of phosphetenes leads to phosphabutadiene derivatives that can further react. Mathey et al. reacted 1,2-dihydrophosphete tungsten pentacarbonyl complexes at high temperatures with dienophiles through [4 + 2] cycloaddition to obtain 6-membered phosphorus heterocycles, through a masked 1-phoshabutadiene intermediate.[22] They found from X-ray crystal structure analysis that the bond between the phosphorus and sp3 carbon is long and weak (1.904 Å), which leads to the equilibrium of the 1,2-dihydrophosphete with 1-phosphabutadiene. Doxsee et al. observed Michael addition to 1,3,4-triphenyl-1,2-dihydrophosphete with various compounds to form 6-membered phosphorus heterocycles as well.[23]
Ring expansion
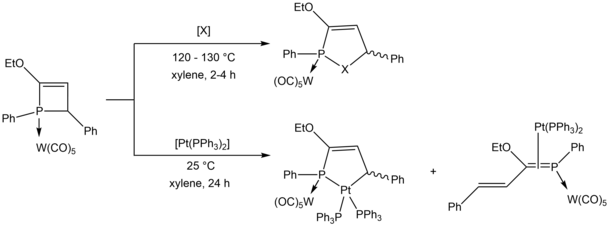
Ring strain in four-membered phosphetene rings can be released by ring expansion to five-membered phosphole rings, which were studied by Mathey et al. for the incorporation of O, S, Se, and Pt[24][25] and Schmidpeter et al. for the incorporation of a phosphorus atom.[16]
References
- ↑ Lauwick, Hortense; Duffy, Matthew P.; Bouit, Pierre-Antoine; Hissler, Muriel (2021-04-15). "Phosphetene: Synthesis and reactivity". Coordination Chemistry Reviews 433: 213759. doi:10.1016/j.ccr.2020.213759. ISSN 0010-8545. https://www.sciencedirect.com/science/article/pii/S0010854520312108.
- ↑ 2.0 2.1 Marinetti, Angela; Fischer, Jean; Mathey, Francois (1985). "Carbonylation of a strained phosphorus-carbon bond. Conversion of phosphirene into 2-keto-1,2-dihydrophosphete complexes: an entry into the chemistry of the phosphorus analogs of unsaturated .beta.-lactams" (in en). Journal of the American Chemical Society 107 (17): 5001–5002. doi:10.1021/ja00303a033. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00303a033.
- ↑ 3.0 3.1 Chen, Hui; Pascal, Simon; Wang, Zuoyong; Bouit, Pierre-Antoine; Wang, Zisu; Zhang, Yinlong; Tondelier, Denis; Geffroy, Bernard et al. (2014). "1,2-Dihydrophosphete: A Platform for the Molecular Engineering of Electroluminescent Phosphorus Materials for Light-Emitting Devices" (in en). Chemistry – A European Journal 20 (31): 9784–9793. doi:10.1002/chem.201400050. ISSN 0947-6539. PMID 24989834. https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.201400050.
- ↑ Leigh, Geoffrey J. (2011). Principles of chemical nomenclature: a guide to IUPAC recommendations. Cambridge: Royal society of chemistry. ISBN 978-1-84973-007-5.
- ↑ Kawashima, Takayuki; Okazaki, Renji (2001-01-01), Mathey, François, ed., "3.2 - Four-membered Rings with 1 Phosphorus Atom", Phosphorus-Carbon Heterocyclic Chemistry (Oxford: Elsevier Science Ltd): pp. 105–165, doi:10.1016/b978-008043952-5/50006-1, ISBN 978-0-08-043952-5, https://www.sciencedirect.com/science/article/pii/B9780080439525500061, retrieved 2023-11-28
- ↑ Eisfeld, Wolfgang; Slany, Michael; Bergsträßer, Uwe; Regitz, Manfred (1994). "Ring enlargement of phosphatriafulvenes with azides to 1H-2-iminophosphetes". Tetrahedron Letters 35 (10): 1527–1530. doi:10.1016/S0040-4039(00)76749-9. ISSN 0040-4039. https://www.sciencedirect.com/science/article/pii/S0040403900767499.
- ↑ Streubel, Rainer; Wilkens, Hendrik; Rohde, Udo; Ostrowski, Annette; Jeske, Jörg; Ruthe, Frank; Jones, Peter G. (1999). "Syntheses, Structures, and Reactions ofC-Methoxycarbonyl-Functionalized Small- and Medium-Sized P-Heterocycle Complexes" (in en). European Journal of Inorganic Chemistry 1999 (9): 1567–1579. doi:10.1002/(SICI)1099-0682(199909)1999:9<1567::AID-EJIC1567>3.0.CO;2-6. ISSN 1434-1948. https://onlinelibrary.wiley.com/doi/10.1002/(SICI)1099-0682(199909)1999:93.0.CO;2-6.
- ↑ Boyd, Bruce A.; Thoma, Randall J.; Neilson, Robert H. (1987). "Oxidation reactions of a 1-phosphadiene: Synthesis of phosphacyclobutenes". Tetrahedron Letters 28 (49): 6121–6124. doi:10.1016/S0040-4039(00)61824-5. ISSN 0040-4039. https://www.sciencedirect.com/science/article/pii/S0040403900618245.
- ↑ Heim, Udo; Pritzkow, Hans; Fleischer, Ulrich; Grützmacher, Hansjörg (1993). "Benzoannulated Phosphorus–Carbon Four-Membered Rings" (in en). Angewandte Chemie International Edition in English 32 (9): 1359–1361. doi:10.1002/anie.199313591. ISSN 0570-0833. https://onlinelibrary.wiley.com/doi/10.1002/anie.199313591.
- ↑ Jun, Hyoung; Angelici, Robert J. (1993). "Mechanism of the platinum-promoted conversion of Cl2C:PR to RC.tplbond.P, where R = 2,4,6-tri-tert-butylphenyl" (in en). Organometallics 12 (11): 4265–4266. doi:10.1021/om00035a003. ISSN 0276-7333. https://pubs.acs.org/doi/abs/10.1021/om00035a003.
- ↑ Streubel, Rainer; Hobbold, Markus; Jeske, Jörg; Jones, Peter G. (1997). "Stereoselective Synthesis and Isomerization of η 1 -2-Phosphabutadiene Complexes to η 1 -2,3-Dihydrophosphete Complexes" (in en). Angewandte Chemie International Edition in English 36 (10): 1095–1097. doi:10.1002/anie.199710951. ISSN 0570-0833. https://onlinelibrary.wiley.com/doi/10.1002/anie.199710951.
- ↑ Marinetti, Angela; Mathey, François (1990). "[2 + 2 Cycloadditions between electron-poor phospha-alkene complexes and electron-rich alkenes or alkynes, a new route to phosphetane and 1,2-dihydrophosphete rings"] (in en). Journal of the Chemical Society, Chemical Communications (2): 153–154. doi:10.1039/c39900000153. ISSN 0022-4936. http://xlink.rsc.org/?DOI=c39900000153.
- ↑ Weber, Lothar; Kaminski, Olaf; Stammler, Hans-Georg; Neumann, Beate; Boese, Roland (1994). "Ubergangsmetall-substituierte Acylphosphane und Phosphaalkene, XXIY [1]. Dipolare [3+2]- und [2+2]-Cycloadditionen von Carbonyl-aktivierten Alkinen an (η5-C5Me5)(CO)2Fe-P=C(NMe2)2. Synthese und Struktur des 1-Phospha-1,3-butadiens (η5-C5Me5)(CO)2Fe-P=C(E)-C(E)=C(NMe2)2(E=CO2Me) / Transition Metal Substituted Acylphosphanes and Phosphaalkenes, XXIV [1]. Dipolar [3+2]- and [2+2]-Cycloadditions of Carbonyl Activated Alkynes to (η5-C5Me5)(CO)2Fe-P=C(NMe2)2. Synthesis and Structure of the 1-Phospha-1,3-butadiene (η5-C5Me5)(CO)2Fe-P=C(E)-C(E)=C(NMe2)2(E=CO2Me)" (in en). Zeitschrift für Naturforschung B 49 (12): 1693–1706. doi:10.1515/znb-1994-1214. ISSN 1865-7117.
- ↑ Link, Manfred; Niecke, Edgar; Nieger, Martin (1994). "Untersuchungen am System Phosphirenimin/Iminophosphan — Insertionsreaktionen und Isomerisierung" (in en). Chemische Berichte 127 (2): 313–319. doi:10.1002/cber.19941270206. ISSN 0009-2940. https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cber.19941270206.
- ↑ Fluck, Ekkehard; Heckmann, Gernot; Rosche, Fred; Westerhausen, Matthias; Gorbunowa, Ekaterina (1995). "Die Reaktion von λ 5 -Diphospheten mit COS und CO 2 Dihydro-λ 5 -Phosphete" (in en). Zeitschrift für anorganische und allgemeine Chemie 621 (9): 1539–1546. doi:10.1002/zaac.19956210918. ISSN 0044-2313. https://onlinelibrary.wiley.com/doi/10.1002/zaac.19956210918.
- ↑ 16.0 16.1 Jochem, Georg; Schmidpeter, Alfred; Nöth, Heinrich (1996). "Diphosphonio Dihydrophosphetide and 1,2-Diphospholide Cations" (in en). Chemistry – A European Journal 2 (2): 221–227. doi:10.1002/chem.19960020215. ISSN 0947-6539. https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.19960020215.
- ↑ Doxsee, Kenneth M.; Shen, Gregory S.; Knobler, Carolyn B. (1989). "Uses of metallacyclobutenes in heterocyclic synthesis. Synthesis and structural characterization of 1,2-dihydrophosphetes" (in en). Journal of the American Chemical Society 111 (25): 9129–9130. doi:10.1021/ja00207a035. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00207a035.
- ↑ Waschbüsch, Klaus; Le Floch, Pascal; Mathey, François (1996). "Synthesis and Chemistry of 2-(Dibromophosphino)-4,5-dimethylphosphinine" (in en). Organometallics 15 (6): 1597–1603. doi:10.1021/om950873f. ISSN 0276-7333. https://pubs.acs.org/doi/10.1021/om950873f.
- ↑ Dupuis, Laurence; Pirio, Nadine; Meunier, Philippe; Igau, Alain; Donnadieu, Bruno; Majoral, Jean-Pierre (1997). "Zirconocen–Benzyme-Mediated Intramolecular Coupling of Bis(alkynyl)phosphane: A Way to Mono- and Tricyclic 1,2-Dihydrophosphetes" (in en). Angewandte Chemie International Edition in English 36 (9): 987–989. doi:10.1002/anie.199709871. ISSN 0570-0833. https://onlinelibrary.wiley.com/doi/10.1002/anie.199709871.
- ↑ Xin, Tiansi; Cummins, Christopher C. (2023-12-06). "Synthesis of Phosphet-2-one Derivatives via Phosphinidene Transfer to Cyclopropenones" (in en). Journal of the American Chemical Society 145 (48): 25989–25994. doi:10.1021/jacs.3c11263. ISSN 0002-7863. PMID 38009595. https://pubs.acs.org/doi/10.1021/jacs.3c11263.
- ↑ Doxsee, Kenneth M.; Hanawalt, Erin M.; Shen, Gregory S.; Weakley, Timothy J. R.; Hope, Hakon; Knobler, Carolyn B. (1991). "Effects of mercuric chloride and pentacarbonyl tungsten coordination on the structure of a 1,2-dihydrophosphete (phosphacyclobutene)" (in en). Inorganic Chemistry 30 (18): 3381–3389. doi:10.1021/ic00018a004. ISSN 0020-1669. https://pubs.acs.org/doi/abs/10.1021/ic00018a004.
- ↑ Huy, Ngoc Hoa Tran; Mathey, François (1988). "1,2-dihydrophosphetes as masked 1-phosphabutadienes". Tetrahedron Letters 29 (25): 3077–3078. doi:10.1016/0040-4039(88)85089-5. ISSN 0040-4039. https://dx.doi.org/10.1016/0040-4039%2888%2985089-5.
- ↑ Doxsee, Kenneth M.; Shen, Gregory S.; Knobler, Carolyn B. (1990). "Reactivity of 1,2-dihydrophosphetes: formation and structural characterization of a formal [4 + 2 cycloadduct"] (in en). Journal of the Chemical Society, Chemical Communications (22): 1649–1650. doi:10.1039/c39900001649. ISSN 0022-4936. http://xlink.rsc.org/?DOI=c39900001649.
- ↑ Marinetti, Angela; Mathey, Francois (1988). "Preliminary chemical study of (2-oxo-1,2-dihydrophosphete(P-W) pentacarbonyltungsten complexes" (in en). Organometallics 7 (3): 633–637. doi:10.1021/om00093a010. ISSN 0276-7333. https://pubs.acs.org/doi/abs/10.1021/om00093a010.
- ↑ Huy, Ngoc Hoa Tran; Mathey, François (1990). "The insertion of sulfur and selenium into the 1,2-dihydrophosphete ring" (in en). Heteroatom Chemistry 1 (1): 33–35. doi:10.1002/hc.520010105. ISSN 1042-7163. https://onlinelibrary.wiley.com/doi/10.1002/hc.520010105.
 |