Chemistry:N,N-Dimethylethylamine
From HandWiki
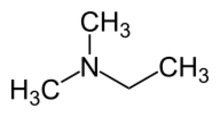
| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Dimethylethanamine | |
| Other names
Ethyl(dimethyl)amine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C4H11N | |
| Molar mass | 73.139 g·mol−1 |
| Appearance | Volatile liquid at room temp. |
| Density | 0.7±0.1 g/cm3 |
| Melting point | −140 °C (−220 °F; 133 K) |
| Boiling point | 36.5 °C (97.7 °F; 309.6 K) |
| Vapor pressure | 495.4±0.1 mmHg |
| Acidity (pKa) | 10.16 (for the conjugate acid) (H2O)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N,N-Dimethylethylamine (DMEA), sometimes referred to as dimethylethylamine, is an organic compound with formula (CH3)2NC2H5. It is an industrial chemical that is mainly used in foundries as a catalyst for epoxy resins and polyurethane as well as sand core production.[2] [3] Dimethylethylamine is a malodorous, volatile liquid at room temperature that is excreted at greater concentrations with larger dietary intake of trimethylamine.[1]
See also
References
- ↑ 1.0 1.1 "N,N-Dimethylethylamine". Toxnet. Hazardous Substance Data Bank. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+5712. Retrieved 4 May 2014. "The aim was to study the effect of trimethylamine (TMA) on the metabolism of the industrial catalyst N,N-dimethylethylamine to ascertain whether biological monitoring of industrial exposure to N,N-dimethylethylamine is compromised and excretion of the malodorous N,N-dimethylethylamine in sweat and urine is increased by dietary intake of TMA....Although the increased urinary and hidrotic excretion of N,N-dimethylethylamine may contribute to body odor problems, they were primarily due to TMA excretion, which is much the greater."
- ↑ Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001.
- ↑ "Dimethylethylamine". BASF The Chemical Company. http://www.basf.com/group/corporate/us/en/brand/N_N_DIMETHYLETHYLAMINE. Retrieved 4 May 2014.
 |

