Chemistry:Lower sulfur oxides
The lower sulfur oxides are a group of inorganic compounds with the formula S
mO
n, where 2m > n. These species are often unstable and thus rarely encountered in everyday life. They are significant intermediates in the combustion of elemental sulfur.[1] Some well characterized examples include sulfur monoxide (SO), its dimer S
2O
2, and a series of cyclic sulfur oxides, S
nO
x (x = 1, 2), based on cyclic S
n rings.
Interest in the lower sulfur oxides has increased because of the need to understand terrestrial atmospheric sulfur pollution and the finding that the extraterrestrial atmospheres of Io, one of Jupiter's moons, and Venus contain significant amounts of sulfur oxides.
Some compounds reported by early workers such as the blue "sesquioxide", S
2O
3, formed by dissolving sulfur in liquid SO
3 appears to be a mixture of polysulfate salts of the S2+
4 and S2+
8 ions.[1]
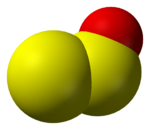
Sulfur monoxide, disulfur dioxide, disulfur monoxide
These species are well characterized in the gas phase, but they cannot be isolated as solids or liquids. Instead, when condensed, they undergo dimerization and oligomerization, usually yielding sulfur dioxide and elemental sulfur. At a few millibars pressure, the relative stabilities are S
2O > S
2O
2 > SO.[2] Sulfur monoxide (SO) and its dimer (S
2O
2) have been trapped at low temperature. Disulfur dioxide (S
2O
2) is a dimer of sulfur monoxide. It has C2v structure (planar).
Disulfur monoxide (S
2O) is an analogue of sulfur dioxide. Like SO
2 as well as ozone (O
3), and trisulfur (S
3), it adopts a bent structure. The S-S bond length is 188.4 pm, the S-O bond is 146.5 pm and the SSO angle is 117.88°. The two dipole moment components are μa = 0.875 D and μb = 1.18 D.[3] This species decomposes to give a polymeric sulfur oxides ("PSO's") with the approximate formula [S
3O]
n. PSO's decompose at room temperature to elemental sulfur and SO
2. PSO's have been proposed to be responsible for the colour of Io.[4]
Trisulfur monoxide, S
3O is an unstable molecule. It has been detected in the gas phase using neutralization-reionization mass spectrometry. Both cyclic and chain structures were found.[5]
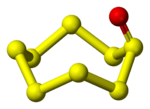
Cyclic SnO
x (x = 1, 2)
A number of monoxides S
nO are known where n = 5-10. The oxygen atom is exocyclic.[2] They can be prepared by oxidising the homocycles with trifluoroperoxyacetic acid:[1]
- S
n + CF
3C(O)OOH → S
nO + CF
3C(O)OH
The compounds are yellow or orange-coloured and thermally unstable near room temperature.[1]
| formula | color (25 °C) | m.p. (°C)[2] |
|---|---|---|
| S 6O |
yellow | 39 |
| S 7O |
orange | 55 |
| S 7O 2 |
intense orange | 60–62 (decomp.) |
| S 8O |
orange | 78 (decomposition) |
| S 9O |
intensely yellow | 32-34 |
| S 10O |
orange | 51 (decomp.) |
One dioxide is well characterized: the deep orange S
7O
2 (m.p. 60–62 °C with decomposition), which arises using trifluoroperoxoacetic acid.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ 2.0 2.1 2.2 R. Steudel (2003). "Sulfur-Rich Oxides SnO and SnO2". in Steudel, R.. Elemental Sulfur und Sulfur-Rich Compounds II. Berlin-Heidelberg: Springer. doi:10.1007/b13185. ISBN 9783540449515.
- ↑ Meschi, D.J.; Myers R.J. (1959). "The microwave spectrum, structure, and dipole moment of disulfur monoxide". Journal of Molecular Spectroscopy 3 (1–6): 405–416. doi:10.1016/0022-2852(59)90036-0. Bibcode: 1959JMoSp...3..405M.
- ↑ Baklouti D., D; Schmitt, B.; Brissaud, O. (November 2004). "Infrared study of lower sulfur oxides on Io's surface". Bulletin of the American Astronomical Society 36: 1099. Bibcode: 2004DPS....36.1607B.
- ↑ de Petris, G; Rosi M Troiani A (2006). "S3O and S3O+ in the gas phase: ring and open-chain structures". Chemical Communications (42): 4416–4418. doi:10.1039/b609646h. PMID 17057862.
 |