Chemistry:Laninamivir
 | |
| Clinical data | |
|---|---|
| Routes of administration | Inhalation |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
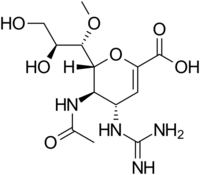
| Formula | C13H22N4O7 |
| Molar mass | 346.340 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Laninamivir (CS-8958) is a neuraminidase inhibitor that is a drug used for the treatment and prophylaxis of Influenzavirus A and Influenzavirus B.[1] It is currently in Phase III clinical trials.[2] It is a long-acting neuraminidase inhibitor administered by nasal inhalation.[3]
Laninamivir was approved for influenza treatment in Japan in 2010 and for prophylaxis in 2013. It is currently marketed under the name Inavir by Daiichi Sankyo. Biota Pharmaceuticals [4] and Daiichi Sankyo co-own Laninamivir. On 1 April 2011, BARDA awarded up to an estimated U$231m to Biota Pharmaceuticals (Formerly Biota Holdings Ltd) wholly owned subsidiary, Biota Scientific Management Pty Ltd, for the advanced development of Laninamivir in the US.[5] It is under clinical evaluations in other countries.[3][6]
References
- ↑ "CS-8958, a prodrug of the new neuraminidase inhibitor R-125489, shows long-acting anti-influenza virus activity". Antimicrobial Agents and Chemotherapy 53 (1): 186–192. January 2009. doi:10.1128/AAC.00333-08. PMID 18955520.
- ↑ "Developing new antiviral agents for influenza treatment: what does the future hold?". Clinical Infectious Diseases. 48 48 Suppl 1 (S1): S3-13. January 2009. doi:10.1086/591851. PMID 19067613.
- ↑ 3.0 3.1 "Influenza virus resistance to neuraminidase inhibitors". Antiviral Research 98 (2): 174–185. May 2013. doi:10.1016/j.antiviral.2013.03.014. PMID 23523943.
- ↑ "Aviragen Therapeutics - Home". http://www.biotapharma.com/.
- ↑ "Biota Pharmaceuticals - Home". http://www.biotapharma.com/?page=1021001&subpage=1021019.
- ↑ "Treatment Guidelines for Influenza Virus Infection: What Does the Recent Guideline State?". Influenza: Advances in Diagnosis and Management. Respiratory Disease Series: Diagnostic Tools and Disease Managements.. Singapore: Springer. 2020. pp. 129–136 (132). doi:10.1007/978-981-15-9109-9_13. ISBN 978-981-15-9109-9. https://books.google.com/books?id=J0UIEAAAQBAJ&dq=Laninamivir+clinical+evaluations+in+other+countries&pg=PA132.
 |


