Chemistry:K-Ras(G12C) inhibitor 6
| |
| Names | |
|---|---|
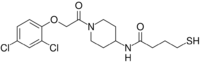
| Preferred IUPAC name
N-{1-[(2,4-Dichlorophenoxy)acetyl]piperidin-4-yl}-4-sulfanylbutanamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C17H22Cl2N2O3S | |
| Molar mass | 405.33 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
K-Ras(G12C) inhibitor 6 is an irreversible inhibitor of oncogenic K-Ras(G12C),[1] subverting the native nucleotide preference to favour GDP over GTP. Its family of inhibitors allosterically control GTP affinity and effector interactions by fitting inside a "pocket", or binding site, of mutant K-Ras. It is the most frequently mutated oncogene.[2]
Investigators and pathologists previously thought that K-Ras is undruggable.[3] However, Kevan M. Shokat and his colleagues, in the Howard Hughes Medical Institute (HHMI) at the University of California, recently reported a novel discovery of "Achilles heel" on K-RAs, and believed that it has real translational implications for patients with K-RAs mutation.[citation needed]
In recent years, significant research efforts have focused on finding effective inhibitors for the Kras-G12C mutation. For instance, sotorasib (Lumakras) became the first FDA-approved targeted therapy for the treatment of patients with NSCLC harboring the Kras-G12C mutation in 2021.[4] Adagrasib (MRTX849) is another inhibitor that has shown promising results in clinical trials.[5]
References
- ↑ "K-Ras(G12C) inhibitor 6". selleckchem.com. http://www.selleckchem.com/products/k-ras-g12c-inhibitor-6.html.
- ↑ "Targeting KRAS-G12C | Amgen Oncology" (in en). https://www.amgenoncology.com/targets/kras.html.
- ↑ "Researchers identify new mechanism to target 'undruggable' cancer gene". www.sciencedaily.com. https://www.sciencedaily.com/releases/2016/04/160421133651.htm.
- ↑ "Sotorasib is First KRAS Inhibitor Approved by FDA - NCI" (in en). 2021-06-25. https://www.cancer.gov/news-events/cancer-currents-blog/2021/fda-sotorasib-lung-cancer-kras.
- ↑ Dhillon, Sohita (February 2023). "Adagrasib: First Approval". Drugs 83 (3): 275–285. doi:10.1007/s40265-023-01839-y. ISSN 1179-1950. PMID 36763320. https://pubmed.ncbi.nlm.nih.gov/36763320/.
 |

