Chemistry:Hydroxytyrosol

| |
| Names | |
|---|---|
| Preferred IUPAC name
4-(2-Hydroxyethyl)benzene-1,2-diol | |
| Other names
3-Hydroxytyrosol
3,4-dihydroxyphenylethanol (DOPET) Dihydroxyphenylethanol 2-(3,4-Di-hydroxyphenyl)-ethanol (DHPE) 3,4-dihydroxyphenolethanol (3,4-DHPEA)[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H10O3 | |
| Molar mass | 154.165 g·mol−1 |
| Appearance | colorless solid |
| 5 g/100 ml | |
| Hazards | |
| Main hazards | Causes skin irritation.
Causes serious eye irritation. May cause respiratory irritation. |
| Safety data sheet | [1] |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Related compounds | |
Related alcohols
|
benzyl alcohol, tyrosol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydroxytyrosol is an organic compound with the formula (HO)
2C
6H
3CH
2CH
2OH. It is a phenylethanoid, i.e. a relative of phenethyl alcohol. Its derivatives are found in a variety of natural sources, notably olive oils and wines. Hydroxytyrosol is a colorless solid,[3][4] although samples often turn beige during storage. It is a derivative, formally speaking, of catechol.
It or its derivatives occurs in olives and in wines[5][6]
Occurrence
Olives
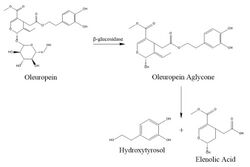
thumb|left|[[Oleuropein, bitter compound, an ester of hydroxytyrosol found in green olive skin]]
The olives, leaves, and olive pulp contain large amounts of hydroxytyrosol derivative Oleuropein, more so than olive oil.[1] Unprocessed, green (unripe) olives, contain between 4.3 and 116 mg of hydroxytyrosol per 100g of olives, while unprocessed, black (ripe) olives contain up to 413.3 mg per 100g.[7] The ripening of an olive substantially increases the amount of hydroxytyrosol.[8] Processed olives, such as the common canned variety containing iron(II) gluconate, contained little hydroxytyrosol, as iron salts are catalysts for its oxidation.[9]
Food safety
Hydroxytyrosol is considered safe as a novel food for human consumption, with a no-observed-adverse-effect level of 50 mg/kg body weight per day, as evaluated by the European Food Safety Authority (EFSA).[10]
In the United States, hydroxytyrosol is considered to be a safe ingredient (GRAS) in processed foods at levels of 5 mg per serving.[11]
Function and production
In nature, hydroxytyrosol is generated by the hydrolysis of oleuropein that occurs during olive ripening. Oleuropein accumulates in olive leaves and fruit as a defense mechanism against pathogens and herbivores. During olive ripening or when the olive tissue is damaged by pathogens, herbivores, or mechanical damage, the enzyme β-glucosidase catalyzes hydroxytyrosol synthesis via hydrolysis from oleuropein.[12]
Metabolism
Shortly after olive oil consumption, 98% of hydroxytyrosol in plasma and urine appears in conjugated forms (65% glucuronoconjugates), suggesting extensive first-past metabolism and a half-life of 2.43 hours.[13]
Mediterranean diet
Mediterranean diets, characterized by regular intake of olive oil, have been shown to positively affect human health, including reduced rates of cardiovascular diseases.[5][14][15] Research on consumption of olive oil and its components includes hydroxytyrosol and oleuropein, which may inhibit oxidation of LDL cholesterol – a risk factor for atherosclerosis, heart attack or stroke.[16] The daily intake of hydroxytyrosol within the Mediterranean diet is estimated to be between 0.15 and 30 mg.[17]
Regulation
Europe
The EFSA has issued a scientific opinion on health claims in relation to dietary consumption of hydroxytyrosol and related polyphenol compounds from olive fruit and oil, and protection of blood lipids from potential oxidative damage.[18]
EFSA concluded that a cause-and-effect relationship existed between the consumption of hydroxytyrosol and related compounds from olives and olive oil and protection of blood lipids from oxidative damage,[18] providing a health claim for consumption of olive oil polyphenols containing at least 5 mg of hydroxytyrosol and its derivatives (oleuropein complex and tyrosol) per 20 g of olive oil.[19]
See also
- Echinacoside, a hydroxytyrosol-containing glycoside
- Tyrosol
- Verbascoside, another hydroxytyrosol-containing glycoside
- Resveratrol
References
- ↑ 1.0 1.1 "Antioxidant activity of tocopherols and phenolic compounds of virgin olive oil". Journal of the American Oil Chemists' Society 73 (11): 1589–1593. 1996. doi:10.1007/BF02523530.
- ↑ "Hydroxytyrosol" (in en). PubChem. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/82755#section=Safety-and-Hazards.
- ↑ "Factors influencing phenolic compounds in table olives (Olea europaea)". Journal of Agricultural and Food Chemistry 60 (29): 7081–7095. July 2012. doi:10.1021/jf3017699. PMID 22720792.
- ↑ "Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health". Molecules 24 (10): 2001. May 2019. doi:10.3390/molecules24102001. PMID 31137753.
- ↑ 5.0 5.1 "Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review". Food Chemistry 130 (4): 797–813. 2012-02-15. doi:10.1016/j.foodchem.2011.08.023. ISSN 0308-8146.
- ↑ "Hydroxytyrosol and its potential therapeutic effects". Journal of Agricultural and Food Chemistry 62 (7): 1449–1455. February 2014. doi:10.1021/jf405820v. PMID 24479643.
- ↑ "Showing all foods in which the polyphenol Hydroxytyrosol is found - Phenol-Explorer". http://phenol-explorer.eu/contents/polyphenol/674.
- ↑ "Table olives and health: a review". Journal of Nutritional Science 9: e57. 2020. doi:10.1017/jns.2020.50. PMID 33354328.
- ↑ "Phenolic compounds change during California-style ripe olive processing". Food Chemistry 74 (1): 55–60. July 2001. doi:10.1016/S0308-8146(00)00338-1.
- ↑ "Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97". EFSA Journal 15 (3): e04728. March 2017. doi:10.2903/j.efsa.2017.4728. PMID 32625437.
- ↑ "GRAS notice for hydroxytyrosol". US Food and Drug Administration. 13 May 2016. https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=600&sort=GRN_No&order=DESC&startrow=1&type=basic&search=hydroxytyrosol.
- ↑ "Factors influencing phenolic compounds in table olives (Olea europaea)". Journal of Agricultural and Food Chemistry 60 (29): 7081–7095. July 2012. doi:10.1021/jf3017699. PMID 22720792.
- ↑ "Hydroxytyrosol disposition in humans". Clinical Chemistry 49 (6 Pt 1): 945–952. June 2003. doi:10.1373/49.6.945. PMID 12765992.
- ↑ "Hydroxytyrosol and its potential therapeutic effects". Journal of Agricultural and Food Chemistry 62 (7): 1449–1455. February 2014. doi:10.1021/jf405820v. PMID 24479643.
- ↑ "The Mediterranean Diet and Cardiovascular Health". Circulation Research 124 (5): 779–798. March 2019. doi:10.1161/CIRCRESAHA.118.313348. PMID 30817261.
- ↑ "Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota". Nutrients 11 (8): 1826. August 2019. doi:10.3390/nu11081826. PMID 31394805.
- ↑ "Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases". Pharmacological Research 143: 58–72. May 2019. doi:10.1016/j.phrs.2019.03.005. PMID 30853597.
- ↑ 18.0 18.1 "Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles" (in en). 8 April 2011. https://www.efsa.europa.eu/en/efsajournal/pub/2033. "From oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006"
- ↑ "EU Register of nutrition and health claims made on foods (v.3.6)". https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/?event=search.
{{Navbox
| name = Neurotransmitter metabolism intermediates | title = Neurotransmitter metabolic intermediates | state = autocollapse| | listclass = hlist
| group1 = catecholamines | list1 = {{Navbox|child
| group1 = Anabolism
(tyrosine→epinephrine) | list1 =
- Tyrosine → Levodopa → [[Chemistry:Dop[[Chemistry:Dopamine → Norepinephrine|Norepinephrine]] → Epinephrine
| group2 = Catabolism/
metabolites
| list2 =
| dopamine: | |
|---|---|
| norepinephrine: | |
| epinephrine: |
}}
| group3 = tryptophan→serotonin| list3 =
| anabolism: | |
|---|---|
| catabolism: |