Chemistry:Gadodiamide
 | |
| Clinical data | |
|---|---|
| Trade names | Omniscan |
| Other names | 2-[bis[2-(carboxylatomethyl-(methylcarbamoylmethyl)amino)ethyl]amino]acetate; gadolinium(+3) cation |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | negligible |
| Metabolism | not metabolized |
| Elimination half-life | 77.8 minutes |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
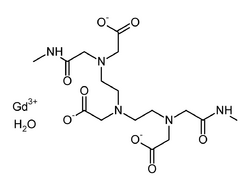
| Formula | C16H28GdN5O9 |
| Molar mass | 591.68 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Gadodiamide, sold under the brand name Omniscan, is a gadolinium-based MRI contrast agent (GBCA), used in magnetic resonance imaging (MRI) procedures to assist in the visualization of blood vessels.
Medical uses
Gadodiamide is a contrast medium used for cranial and spinal magnetic resonance imaging (MRI) and for general MRI of the body after intravenous administration. It provides contrast enhancement and facilitates visualisation of abnormal structures or lesions in various parts of the body including the central nervous system (CNS). It crosses intact the blood brain barrier.[3]
Adverse effects
It is one of the main GBCA associated with nephrogenic systemic fibrosis (NSF), a toxic reaction occurring in some people with kidney problems.[4] No cases have been seen in people with normal kidney function.[5]
A 2015 study found gadolinium deposited in the brain tissue of people who had received gadodiamide.[6] Other studies using post-mortem mass spectrometry found most of the deposit remained at least 2 years after an injection and deposit also in individuals with no kidney issues.
In vitro studies found it to be neurotoxic.[7]
An Italian task force recommended that breastfeeding mothers precautionally avoid any contrast agent such as gadodiamide that has been associated with nephrogenic systemic fibrosis.[8]
Society and culture
Gadodiamide was suspended along with gadopentetic acid (Magnevist) by the European Medicines Agency in 2017.[9]
References
- ↑ "Omniscan- gadodiamide injection". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1e9a37e2-f28a-4373-bf0f-3e9b60f42d8a.
- ↑ "Active substance: gadodiamide". List of nationally authorised medicinal products. European Medicine Agency. 14 January 2021. https://www.ema.europa.eu/documents/psusa/gadodiamide-list-nationally-authorised-medicinal-products-psusa/00001503/202004_en.pdf.
- ↑ "Retention of Gadolinium in Brain Parenchyma: Pathways for Speciation, Access, and Distribution. A Critical Review". Journal of Magnetic Resonance Imaging 52 (5): 1293–1305. 2020. doi:10.1002/jmri.27124. PMID 32246802.
- ↑ "Magnetic Resonance Imaging (MRI), Gadolinium". StatPearls [Internet] (Treasure Island (FL): StatPearls Publishing). January 2018. PMID 29494094.
- ↑ "Gadolinium-associated nephrogenic systemic fibrosis: the need for nephrologists' awareness". Journal of Nephrology 21 (3): 324–36. 2008. PMID 18587720.
- ↑ "Gadolinium Found in Brain Tissue". Medscape. 26 March 2015. http://www.medscape.com/viewarticle/842107.
- ↑ "Gadolinium-Based MRI Contrast Agents Induce Mitochondrial Toxicity and Cell Death in Human Neurons, and Toxicity Increases With Reduced Kinetic Stability of the Agent". Investigative Radiology 54 (8): 453–463. August 2019. doi:10.1097/RLI.0000000000000567. PMID 31265439. https://boris.unibe.ch/132314/.
- ↑ "Radiological contrast media in the breastfeeding woman: a position paper of the Italian Society of Radiology (SIRM), the Italian Society of Paediatrics (SIP), the Italian Society of Neonatology (SIN) and the Task Force on Breastfeeding, Ministry of Health, Italy". European Radiology 24 (8): 2012–22. August 2014. doi:10.1007/s00330-014-3198-6. PMID 24838733.
- ↑ "Gadolinium-containing contrast agents: removal of Omniscan and iv Magnevist, restrictions to the use of other linear agents". 14 December 2017. https://www.gov.uk/drug-safety-update/gadolinium-containing-contrast-agents-removal-of-omniscan-and-iv-magnevist-restrictions-to-the-use-of-other-linear-agents.
External links
- "Gadodiamide". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/gadodiamide.
 |


