Chemistry:Ethyl chloroformate
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Ethyl carbonochloridate | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1182 |
| |
| |
| Properties | |
| ClCO 2CH 2CH 3 | |
| Molar mass | 108.52 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Like hydrochloric acid[2] |
| Density | 1.1403 g/cm3 |
| Melting point | −81 °C (−114 °F; 192 K) |
| Boiling point | 95 °C (203 °F; 368 K) |
| Decomposes | |
| Hazards | |
| Main hazards | Corrosive Flammable |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H225, H302, H314, H330 | |
| P210, P233, P240, P241, P242, P243, P260, P264, P270, P271, P280, P284, P301+312, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P320, P321, P330, P363, P370+378, P403+233, P403+235 | |
| NFPA 704 (fire diamond) | |
| Flash point | 61 °C (142 °F; 334 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
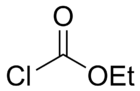
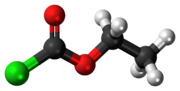
Ethyl chloroformate is an organic compound with the chemical formula ClCO
2CH
2CH
3. It is the ethyl ester of chloroformic acid. It is a colorless, corrosive and highly toxic liquid. It is a reagent used in organic synthesis for the introduction of the ethyl carbamate protecting group[3] and for the formation of carboxylic anhydrides.
Preparation
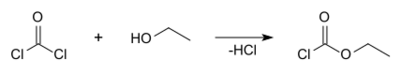
Ethyl chloroformate can be prepared using ethanol and phosgene:
Safety
Ethyl chloroformate is a highly toxic, flammable, corrosive substance. It causes severe burns when comes in contact with eyes and/or skin, can be harmful if swallowed or inhaled.[4]
References
- ↑ Merck Index, 11th Edition, 3742.
- ↑ https://www.sigmaaldrich.com/GB/en/sds/aldrich/185892
- ↑ Protective Groups in Organic Synthesis, Third Edition, Theodora W. Greene and Peter G. M. Wuts, pages 504-506, ISBN:0-471-16019-9
- ↑ PubChem. "Ethyl chloroformate" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/10928.
 |