Chemistry:Disulfur dibromide
From HandWiki
| |
 Sulfur, S Bromine, Br | |

| |
| Names | |
|---|---|
| IUPAC name
Dibromodisulfane
| |
| Other names
Bromosulfanyl thiohypobromite
Disulfur dibromide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| S 2Br 2 | |
| Molar mass | 223.93 g·mol−1 |
| Appearance | Orange/yellow liquid |
| Density | 2.703 g/cm3 |
| Boiling point | 46–48 °C (115–118 °F; 319–321 K) (0.1 mmHg) |
| Structure | |
| C2 | |
| 2 at sulfur atoms | |
| gauche | |
| Hazards | |
| Safety data sheet | ICSC 1661 |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| Related compounds | |
Related
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Disulfur dibromide is the inorganic compound with the formula S
2Br
2. It is a yellow-brown liquid that fumes in air. It is prepared by direct combination of the elements and purified by vacuum distillation.[1] The compound has no particular application, unlike the related sulfur compound disulfur dichloride.
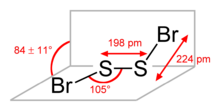
The molecular structure is Br–S–S–Br, akin to that of disulfur dichloride (S
2Cl
2). According to electron diffraction measurements, the angle between the Bra
–S–S and S–S–Brb
planes is 84° and the Br–S–S angle is 107°. The S–S distance is 198.0 pm, circa 5.0 pm shorter than for S
2Cl
2.[2]
References
- ↑ F. Fehér (1963). "Dibromodisulfane". in G. Brauer. Handbook of Preparative Inorganic Chemistry, 2nd Ed.. 1. NY, NY: Academic Press. pp. 377–378.
- ↑ Zysman-Colman, Eli; Harpp, David (2004). "Comparison of the Structural Properties of Compounds Containing the XSSX Moiety (X = H, Me, R, Cl, Br, F, OR)". Journal of Sulfur Chemistry 25: 291-316. doi:10.1080/17415990410001710163.
 |

