Chemistry:Dimethylzinc
 | |
| Names | |
|---|---|
| IUPAC name
Dimethylzinc
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Zn(CH 3) 2 | |
| Molar mass | 95.478 g/mol |
| Appearance | Colorless liquid |
| Odor | Garlic[1] |
| Density | 1.386 g/cm3 at 10.5 °C[1] |
| Melting point | −42 °C (−44 °F; 231 K) |
| Boiling point | 46 °C (115 °F; 319 K) |
| Solubility | Soluble in xylene, diethyl ether, hydrocarbons; decomposes in water, ethanol and acids[1] |
| Vapor pressure | 50.13 kPa[1] |
| Thermal conductivity | 0.1627 W/(m∙K) at 70 °C (158 °F)[1] |
| Viscosity | 0.807 mPa·s at 70 °F (21 °C)[1] |
| Thermochemistry | |
Heat capacity (C)
|
129.20 J/(mol∙K) ((liquid at 25 °C (77 °F))[2] |
| Hazards | |
| Main hazards | Spontaneously ignites in air and violently reacts with water, evolving irritant and toxic fumes.[1] |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| HH225Script error: No such module "Preview warning".Category:GHS errors, HH250Script error: No such module "Preview warning".Category:GHS errors, HH260Script error: No such module "Preview warning".Category:GHS errors, HH314Script error: No such module "Preview warning".Category:GHS errors, HH410Script error: No such module "Preview warning".Category:GHS errors | |
| PP210Script error: No such module "Preview warning".Category:GHS errors, PP222Script error: No such module "Preview warning".Category:GHS errors, PP223Script error: No such module "Preview warning".Category:GHS errors, PP231+P232Script error: No such module "Preview warning".Category:GHS errors, PP233Script error: No such module "Preview warning".Category:GHS errors, PP235Script error: No such module "Preview warning".Category:GHS errors, PP240Script error: No such module "Preview warning".Category:GHS errors, PP241Script error: No such module "Preview warning".Category:GHS errors, PP242Script error: No such module "Preview warning".Category:GHS errors, PP243Script error: No such module "Preview warning".Category:GHS errors, PP260Script error: No such module "Preview warning".Category:GHS errors, PP264Script error: No such module "Preview warning".Category:GHS errors, PP273Script error: No such module "Preview warning".Category:GHS errors, PP280Script error: No such module "Preview warning".Category:GHS errors, PP301+P330+P331Script error: No such module "Preview warning".Category:GHS errors, PP302Script error: No such module "Preview warning".Category:GHS errors, PP303+P361+P353Script error: No such module "Preview warning".Category:GHS errors, PP304+P340Script error: No such module "Preview warning".Category:GHS errors, PP305Script error: No such module "Preview warning".Category:GHS errors, PP316Script error: No such module "Preview warning".Category:GHS errors, PP317Script error: No such module "Preview warning".Category:GHS errors, PP321Script error: No such module "Preview warning".Category:GHS errors, PP334Script error: No such module "Preview warning".Category:GHS errors, PP335Script error: No such module "Preview warning".Category:GHS errors, PP338Script error: No such module "Preview warning".Category:GHS errors | |
| NFPA 704 (fire diamond) | |
| 0 °F (−18 °C)[1] | |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dimethylzinc, also known as zinc methyl, DMZ, or DMZn, is an organozinc compound with the chemical formula Zn(CH
3)
2. It belongs to the large series of similar compounds such as diethylzinc.
Preparation
It is formed by the action of methyl iodide on zinc or zinc-sodium alloy at elevated temperatures.
- 2 Zn + 2 CH
3I → Zn(CH
3)
2 + ZnI
2
Sodium assists the reaction of the zinc with the methyl iodide. Zinc iodide is formed as a byproduct.
Properties
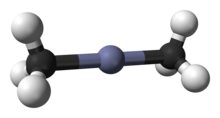
Dimethylzinc is a colorless mobile volatile liquid, which has a characteristic disagreeable garlic-like odor. It is a very reactive and strong reducing agent.[1] It is soluble in alkanes and often sold as a solution in hexanes. The triple point of dimethylzinc is 230.13 K (−43.02 °C) ± 0.02 K.[2] The monomeric molecule of dimethylzinc is linear at Zn center and tetragonal at C centers.
Toxicity and hazards
Inhalation of dimethylzinc mist or vapor causes immediate irritation of the upper respiratory tract, and may cause pneumonia and death. Eyes are immediately and severely irritated and burned by liquid, vapor, or dilute solutions. If not removed by thorough flushing with water, this chemical may permanently damage the cornea, eventually causing blindness. If dimethylzinc contacts the skin, it causes thermal and acid burns by reacting with moisture on skin. Unless washed quickly, skin may be scarred. Ingestion, while unlikely, also causes immediate burns. Nausea, vomiting, cramps, and diarrhea may follow, and tissues may ulcerate if not promptly treated. Upon heating, dimethylzinc vapor decomposes to irritating and toxic products.[1]
Contact of dimethylzinc with oxidants may form explosive peroxides. Dimethylzinc oxidises in air very slowly, producing methylzinc methoxide CH
3ZnOCH
3.
Dimethylzinc is very pyrophoric and can spontaneously ignite in air. It burns in air with a blue flame, giving off a garlic-like odor. The products of decomposition (fire smoke) include zinc oxide, which itself is not toxic, but its fumes can irritate lungs and cause metal fume fever, severe injury, or death.
Dimethylzinc fire must be extinguished with dry sand. The fire reacts violently or explosively with water, generating very flammable methane gas which can explode in air upon catching fire, and lung-irritating smoke of zinc oxide. Dimethylzinc fire reacts violently or explosively with methanol, ethanol and 2,2-dichloropropane. It explodes in oxygen and ozone. Improperly handled containers of dimethylzinc can explode, causing serious injuries or death.[1]
Structure
In the solid state the compound exists in two modifications. The tetragonal high-temperature phase shows a two-dimensional disorder, while the low-temperature phase which is monoclinic is ordered. The molecules are linear with Zn-C bond lengths measuring 192.7(6) pm.[3] The structure of the gas-phase shows a very similar Zn-C distance of 193.0(2) pm.[4]
History
Dimethylzinc was first prepared by Edward Frankland during his work with Robert Bunsen in 1849 at the University of Marburg. After heating a mixture of zinc and methyl iodide in an airtight vessel, a flame burst out when the seal was broken.[5] In the laboratory, this synthesis method remains unchanged today, except that copper or copper compounds are used to activate the zinc.
Uses
Dimethylzinc has been of great importance in the synthesis of organic compounds. It was used for a long time to introduce methyl groups into organic molecules or to synthesize organometallic compounds containing methyl groups. Grignard reagents, (organo-magnesium compounds), which are easier to handle and less flammable, replaced organo-zinc compounds in most laboratory syntheses. Due to differences in reactivity (as well as in reaction byproducts) between organo-zinc compounds and Grignard reagents, organo-zinc compounds may be preferred in some syntheses.[6]
Its high vapor pressure has led to extensive uses in the production of semiconductors, e.g. metalorganic chemical vapor deposition (MOCVD) for the preparation of wide band gap II–VI semiconducting films (e.g. ZnO, ZnS, ZnSe, ZnTe, Cd {x}Hg {−
1x}Te) and as p-dopant precursors for III–V semiconductors (e.g. AlN, AlP, Al {x}Ga {−
1x}As, GaAs, InP), which have many electronic and photonic applications.[7]
It is used as an accelerator in rubber vulcanization, as a fungicide, and as a methylating agent in methyltitanium trichloride.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "Dimethylzinc". https://pubchem.ncbi.nlm.nih.gov/compound/Dimethylzinc.
- ↑ 2.0 2.1 "Dimethylzinc (CAS 544-97-8)". https://www.chemeo.com/cid/15-221-9/dimethylzinc.
- ↑ John Bacsa; Felix Hanke; Sarah Hindley; Rajesh Odedra; George R. Darling; Anthony C. Jones; Alexander Steiner (2011). "The Solid State Structures of Dimethylzinc and Diethylzinc". Angewandte Chemie International Edition 50 (49): 11685–11687. doi:10.1002/anie.201105099. PMID 21919175.
- ↑ A. Haaland; J. C. Green; G. S. McGrady; A. J. Downs; E. Gullo; M. J. Lyall; J. Timberlake; A. V. Tutukin et al. (2003). "The length, strength and polarity of metal–carbon bonds: dialkylzinc compounds studied by density functional theory calculations, gas electron diffraction and photoelectron spectroscopy". Dalton Transactions (22): 4356–4366. doi:10.1039/B306840B.
- ↑ E. Frankland (1849). "Notiz über eine neue Reihe organischer Körper, welche Metalle, Phosphor u. s. w. enthalten". Liebigs Annalen der Chemie und Pharmacie 71 (2): 213–216. doi:10.1002/jlac.18490710206. https://zenodo.org/record/1427026.
- ↑ Erdik, Ender (1996). Organozinc reagents in organic synthesis. Boca Raton: CRC Press. ISBN 978-0-8493-9151-4.
- ↑ Mohammad Afzaal; Mohammad A. Malik; Paul O’Brien (2007). "Preparation of zinc containing materials". New Journal of Chemistry 31 (12): 2029–2040. doi:10.1039/b712235g.
 |


