Chemistry:Curium(III) iodide
From HandWiki
| |
| Names | |
|---|---|
| Other names
Curium triiodide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| CmI3 | |
| Molar mass | 628 g·mol−1 |
| Appearance | white crystals |
| Related compounds | |
Related compounds
|
Americium triiodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Curium(III) iodide is the chemical compound with the formula CmI
3.[1][2][3] Since all isotopes of curium are only artificially produced, the compound has no natural occurrence.
Synthesis
Elemental curium and iodine can be reacted to synthesize curium(III) iodide.[4]
- 2Cm + 3I
2 → 2CmI
3
- 2Cm + 3I
Also by the reaction of curium(III) chloride with ammonium iodide:[5][6]
- CmCl
3 + 3NH
4I → CmI
3 + 3NH
4Cl
- CmCl
Physical properties
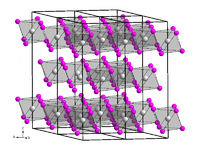
Curium(III) iodide is a colorless ionic compound consisting of Cm3+ and I− ions. It forms white crystals the hexagonal crystal system in the space group R3 (space group no. 148) with the lattice parameters a = 744 pm and c = 2040 pm with six units per unit cell. Its crystal structure is isotypic with that of bismuth(III) iodide.[5][7]
References
- ↑ Lumetta, Gregg J.; Thompson, Major C.; Penneman, Robert A.; Eller, P. Gary (2006). "Curium" (in en). The Chemistry of the Actinide and Transactinide Elements. Springer Netherlands. pp. 1397–1443. doi:10.1007/1-4020-3598-5_9. ISBN 978-1-4020-3598-2. https://link.springer.com/chapter/10.1007/1-4020-3598-5_9. Retrieved 3 July 2023.
- ↑ Brown, David; Canterford, J. H.; Colton, Ray (1968) (in en). Halides of the Transition Elements: Halides of the lanthanides and actinides, by D. Brown. Wiley. p. 260. ISBN 978-0-470-10840-6. https://books.google.com/books?id=EHZMAAAAYAAJ&q=curium+triiodide. Retrieved 3 July 2023.
- ↑ "WebElements Periodic Table » Curium » curium triiodide". winter.group.shef.ac.uk. https://winter.group.shef.ac.uk/webelements/compounds/curium/curium_triiodide.html.
- ↑ Seaborg, G. T.; James, R. A.; Ghiorso, A. (1949). "The Transuranium Elements". Science (McGraw-Hill) 104 (2704): 1554–1571. https://www.jstor.org/stable/1675046. Retrieved 3 July 2023.
- ↑ 5.0 5.1 Asprey, L. B.; Keenan, T. K.; Kruse, F. H. (July 1965). "Crystal Structures of the Trifluorides, Trichlorides, Tribromides, and Triiodides of Americium and Curium" (in en). Inorganic Chemistry 4 (7): 985–986. doi:10.1021/ic50029a013. ISSN 0020-1669. https://digital.library.unt.edu/ark:/67531/metadc1035960/m2/1/high_res_d/4582276.pdf. Retrieved 3 July 2023.
- ↑ Koch, Günter (5 October 2013) (in de). Transurane: Teil C: Die Verbindungen. Springer-Verlag. p. 154. ISBN 978-3-662-11547-3. https://books.google.com/books?id=WCi2BgAAQBAJ&dq=curium+triiodide&pg=PA154. Retrieved 3 July 2023.
- ↑ Macintyre, Jane E. (23 July 1992) (in en). Dictionary of Inorganic Compounds. CRC Press. p. 3046. ISBN 978-0-412-30120-9. https://books.google.com/books?id=9eJvoNCSCRMC&dq=curium+triiodide&pg=PA3046. Retrieved 3 July 2023.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

