Chemistry:Coelenterazine
| |
| Names | |
|---|---|
| IUPAC name
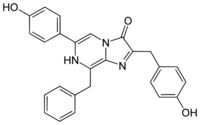
6-(4-Hydroxyphenyl)-2-[(4-hydroxyphenyl)methyl]-8-(phenylmethyl)-7H-imidazo[1,2-a]pyrazin-3-one
| |
| Other names
Renilla luciferin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C26H21N3O3 | |
| Molar mass | 423.472 g·mol−1 |
| Appearance | Orange-yellow crystals |
| Melting point | 175 to 178 °C (347 to 352 °F; 448 to 451 K) |
| Absorbance | ε435 = 9800 M−1 cm−1 (methanol)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Coelenterazine is a luciferin, a molecule that emits light after reaction with oxygen, found in many aquatic organisms across eight phyla.[1] It is the substrate of many luciferases such as Renilla reniformis luciferase (Rluc), Gaussia luciferase (Gluc), and photoproteins, including aequorin, and obelin. All these proteins catalyze the oxidation of this substance, a reaction catalogued EC 1.13.12.5.
History
Coelenterazine was simultaneously isolated and characterized by two groups studying the luminescent organisms sea pansy (Renilla reniformis) and the cnidarian Aequorea victoria, respectively.[2][3] Both groups independently discovered that the same compound was used in both luminescent systems. The molecule was named after the now-obsolete phylum coelenterata. Likewise, the two main metabolites – coelenteramide and coelenteramine – were named after their respective functional groups. While coelenterazine was first discovered in Aequorea victoria, it was later shown that they do not synthesize coelenterazine, but obtain it through their diet, largely from crustaceans and copepods.[4]
Occurrence
Coelenterazine is widely found in marine organisms including:
- radiolarians
- ctenophores
- cnidarians such as Aequorea victoria, Obelia geniculata and Renilla reniformis
- squid such as Watasenia scintillans and Vampyroteuthis infernalis
- shrimp such as Systellaspis debilis and Oplophorus gracilirostris
- copepods such as Pleuromamma xiphias and Gaussia princeps
- chaetognaths[5]
- fish including some Neoscopelidae and Myctophidae
- echinoderms such as Amphiura filiformis
The compound has also been isolated from organisms that are not luminescent, such as the Atlantic herring and several shrimp species including Pandalus borealis and Pandalus platyuros.
Biosynthesis
Biosynthesis of coelenterazine in Metridia starts from two molecules of tyrosine and one molecule of phenylalanine, and some researchers believe this comes in the form of a cyclized "Phe-Tyr-Tyr" (FYY) peptide.[6]
Many members of the genus Metridia also produce luciferases that use this compound,[7] some of which are secreted into extracellular space, an unusual property for luciferases.[8]
Properties
Coelenterazine can be crystallized into orange-yellow crystals. The molecule absorbs light in the ultraviolet and visible spectrum, with peak absorption at 435 nm in methanol, giving the molecule a yellow color. The molecule spontaneously oxidizes in aerobic conditions or in some organic solvents such as dimethylformamide and DMSO and is preferentially stored in methanol or with an inert gas.
Synthetic coelenterazine derivatives
To improve its biophysical properties, derivatives of coelenterazine have been synthesized by means of different procedures including multicomponent strategies.[9]
See also
- Coelenteramide
- Coelenteramine
- Furimazine
- Vargulin
References
- ↑ 1.0 1.1 Shimomura, O. (2006). Bioluminescence: Chemical Principles and Methods. World Scientific Publishing. pp. 159–65. ISBN 978-981-256-801-4. https://archive.org/details/bioluminescencec00shim_065.
- ↑ "Structure of native Renilla reinformis luciferin". Proceedings of the National Academy of Sciences of the United States of America 74 (10): 4285–7. October 1977. doi:10.1073/pnas.74.10.4285. PMID 16592444. Bibcode: 1977PNAS...74.4285H.
- ↑ "Chemical nature of bioluminescence systems in coelenterates". Proceedings of the National Academy of Sciences of the United States of America 72 (4): 1546–9. April 1975. doi:10.1073/pnas.72.4.1546. PMID 236561. Bibcode: 1975PNAS...72.1546S.
- ↑ "Can coelenterates make coelenterazine? Dietary requirement for luciferin in cnidarian bioluminescence". Proceedings of the National Academy of Sciences of the United States of America 98 (20): 11148–51. September 2001. doi:10.1073/pnas.201329798. PMID 11572972. Bibcode: 2001PNAS...9811148H.
- ↑ "A bioluminescent chaetognath". Nature 367 (6460): 225–26. 1994. doi:10.1038/367225a0. Bibcode: 1994Natur.367..225H. http://www.lifesci.ucsb.edu/~haddock/abstracts/haddock_chaeto.pdf. Retrieved 2008-10-28.
- ↑ "Occurrence of Isopenicillin-N-Synthase Homologs in Bioluminescent Ctenophores and Implications for Coelenterazine Biosynthesis". PLOS ONE 10 (6): e0128742. 30 June 2015. doi:10.1371/journal.pone.0128742. PMID 26125183. Bibcode: 2015PLoSO..1028742F.
- ↑ "Metridia lucens". PeerJ 6: e5506. 14 September 2018. doi:10.7717/peerj.5506. PMID 30233994.
- ↑ "Cloning and expression of cDNA for a luciferase from the marine copepod Metridia longa. A novel secreted bioluminescent reporter enzyme". The Journal of Biological Chemistry 279 (5): 3212–7. January 2004. doi:10.1074/jbc.M309639200. PMID 14583604.
- ↑ "Multicomponent Synthesis of Novel Coelenterazine Derivatives Substituted at the C-3 Position". Tetrahedron 71 (46): 8781–85. 2015. doi:10.1016/j.tet.2015.09.048.
External links
- Bioluminescence Page showing major luciferin types.
External links
 |

