Chemistry:Carboxyatractyloside
| |
| Names | |
|---|---|
| IUPAC name
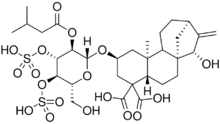
15α-Hydroxy-2β-[2-O-(3-methylbutanoyl)-3,4-di-O-sulfono-β-D-glucopyranosyloxy]-5β,8α,9β,10α,13α-kaur-16-ene-18,19-dioic acid
| |
| Systematic IUPAC name
(2S,4aS,6aR,7S,9R,11aS,11bS)-7-Hydroxy-2-({(2R,3R,4R,5R,6R)-6-(hydroxymethyl)-3-[(3-methylbutanoyl)oxy]oxan-2-yl}oxy)-11b-methyl-8-methylidenedodecahydro-6a,9-methanocyclohepta[a]naphthalene-4,4(1H)-dicarboxylic acid | |
| Other names
CATR
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| DrugBank | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C31H46O18S2 | |
| Molar mass | 770.81 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Carboxyatractyloside (CATR) is a highly toxic diterpene glycoside that inhibits the ADP/ATP translocase. It is about 10 times more potent than its analog atractyloside.[1] While atractyloside is effective in the inhibition of oxidative phosphorylation, carboxyatractyloside is considered to be more effective.[2] The effects of carboxyatractyloside on the ADP/ATP translocase are not reversed by increasing the concentration of adenine nucleotides, unlike its counterpart atractyloside.[2] Carboxyatractyloside behavior resembles bongkrekic acid while in the mitochondria.[2] Carboxyatractyloside is poisonous to humans as well as livestock, including cows[3] and horses.[4]
Symptoms of carboxyatractyloside poisoning may include abdominal pain, nausea and vomiting, drowsiness, palpitations, sweating and trouble breathing.[5] In severe cases, convulsions, liver failure and loss of consciousness may develop, which can lead to death.[5]
Carboxyatractyloside can be found in Xanthium species plants, including Xanthium strumarium.[6] Consumption of Xanthium containing the toxin led to the deaths of at least 19 people in Sylhet, Bangladesh during a period of food scarcity.[7] Along with atractyloside, it is also one of the main poisonous substances in the Atractylis gummifera thistle.[8]
References
- ↑ "Probing the interactions of carboxy-atractyloside and atractyloside with the yeast mitochondrial ADP/ATP carrier". Structure 18 (1): 39–46. January 2010. doi:10.1016/j.str.2009.11.009. PMID 20152151.
- ↑ 2.0 2.1 2.2 "Effects of carboxyatractyloside a structural analogue of atractyloside on mitochondrial oxidative phosphorylation". Life Sciences 10 (17, Pt. 2): 961–8. September 1971. doi:10.1016/0024-3205(71)90099-3. PMID 4255019. https://pubmed.ncbi.nlm.nih.gov/4255019/.
- ↑ "Analytical confirmation of Xanthium strumarium poisoning in cattle". J Vet Diagn Invest 26 (5): 640–645. September 2014. doi:10.1177/1040638714542867. PMID 25012081.
- ↑ Wilson, DA (2011). "Cocklebur Toxicosis". Clinical Veterinary Advisor - The Horse. University of Missouri, Columbia, Missouri: Elsevier. p. 115-116. ISBN 978-1-4160-9979-6. https://www.sciencedirect.com/book/9781416099796/clinical-veterinary-advisor.
- ↑ 5.0 5.1 "Carboxyatractyloside poisoning in humans". Annals of Tropical Medicine and Public Health 25 (2): 125–134. June 2005. doi:10.1179/146532805X45728. PMID 15949201. https://pubmed.ncbi.nlm.nih.gov/15949201/. Retrieved 1 January 2021.
- ↑ Plumlee, Konnie (2004). "Chapter 25 - Plants". Clinical Veterinary Toxicology. Mosby. ISBN 978-0-323-01125-9. https://www.sciencedirect.com/science/article/pii/B032301125X500285.
- ↑ "Fatal outbreak from consuming Xanthium strumarium seedlings during time of food scarcity in northeastern Bangladesh". PLOS ONE 5 (3): e9756. March 2010. doi:10.1371/journal.pone.0009756. PMID 20305785. Bibcode: 2010PLoSO...5.9756G.
- ↑ "Atractylis gummifera L. poisoning: an ethnopharmacological review". Ethnopharmacol. 97 (3): 175–181. February 2005. doi:10.1016/j.jep.2004.11.025. PMID 15707749. https://pubmed.ncbi.nlm.nih.gov/15707749/.
 |

