Chemistry:Bis(trimethylsilyl) peroxide
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C6H18O2Si2 | |
| Molar mass | 178.378 g·mol−1 |
| Appearance | colorless oil |
| Density | 0.829 g/cm3 |
| Boiling point | 35 °C (95 °F; 308 K) 35 torr |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
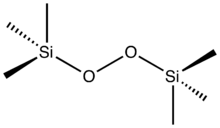
Bis(trimethylsilyl) peroxide (sometimes abbreviated as BTSP)[1] is an organosilicon compound with the formula ((CH3)3SiO)2. It is a colorless liquid that is soluble in organic solvents so long as they lack acidic groups. The compound represents an aprotic analogue of hydrogen peroxide and as such it is used for certain sensitive organic oxidations.[2] Upon treatment with organolithium compounds, it affords the silyl ether.
Preparation
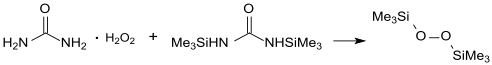
It is prepared by treating trimethylsilyl chloride with anhydrous adducts of hydrogen peroxide, e.g. Hydrogen peroxide-urea complex.[3]
References
- ↑ Baj, Stefan; Chrobok, Anna; Słupska, Roksana (2009-02-01). "The Baeyer–Villiger oxidation of ketones with bis(trimethylsilyl) peroxide in the presence of ionic liquids as the solvent and catalyst" (in en). Green Chemistry 11 (2): 279–282. doi:10.1039/B814534B. ISSN 1463-9270.
- ↑ Ishikawa, Hayato; Elliott, Gregory I.; Velcicky, Juraj; Choi, Younggi; Boger, Dale L. (2006). "Total Synthesis of (−)- andent-(+)-Vindoline and Related Alkaloids". Journal of the American Chemical Society 128 (32): 10596–10612. doi:10.1021/ja061256t. PMID 16895428.
- ↑ Jih Ru Hwu, Buh-Luen Chen, Santhosh F. Neelamkavil, Yuzhong Chen (2002). "Encyclopedia of Reagents for Organic Synthesis". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rb219.pub3. ISBN 0471936235.