Chemistry:Benzoquinonetetracarboxylic acid
| |
| Names | |
|---|---|
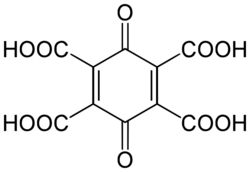
| Preferred IUPAC name
3,6-Dioxocyclohexa-1,4-diene-1,2,4,5-tetracarboxylic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C10H4O10 | |
| Molar mass | 284.14 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In chemistry, 1,4-benzoquinonetetracarboxylic acid is an organic compound with formula C10H4O10, or (C6O2)(-(CO)OH)4, which can be viewed as deriving from para-benzoquinone C6H4O2 through replacement of the four hydrogen atoms by carboxyl functional groups -(CO)OH.
By removal of four protons, the acid is expected to yield the anion C10O4−10, benzoquinonetetracarboxylate, which is one of the oxocarbon anions (consisting solely of oxygen and carbon). By loss of 1 through 3 protons, it forms the anions C10H3O−10, C10H2O2−10, and C10HO3−10, called respectively trihydrogen-, dihydrogen-, and hydrogenbenzoquinonetetracarboxylate. The same names are used for the corresponding esters.
Removal of two water molecules gives the compound benzoquinonetetracarboxylic dianhydride, C10O8, one of the oxides of carbon.[1]
The acid can be obtained by from durene (1,2,4,5-tetramethylbenzene) via dinitropyromellitic and diaminopyromellitic acids.[2][3][4]
See also
- Mellitic acid C12H6O12
- Tetrahydroxybenzoquinone C6H4O6
- Benzenehexol C6H6O6
References
- ↑ P. R. Hammond (1963), 1,4-Benzoquinone Tetracarboxylic Acid Dianhydride, C10O8: A Strong Acceptor. Science, Vol. 142. no. 3591, p. 502 doi:10.1126/science.142.3591.502
- ↑ B. I. Zapadinskii, B. I. Liogon'kii, and A. A. Berlin (1973), Syntheses of Tetracarboxylic Acids. Russian Chemical Reviews, volume 42 issue 11, page 939. Online version accessed on 2010-01-03.
- ↑ J. U. Nef (1887), Annalen, volume 237, page 19. Cited by Zapadinskii et al.
- ↑ J. U. Nef (1890), Annalen, volume 258, page 282. Cited by Zapadinskii et al.
 |

