Chemistry:Azaribine
From HandWiki
Short description: Chemical compound
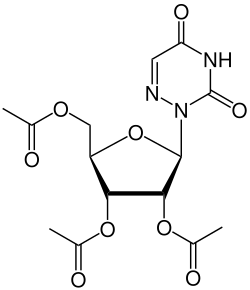 | |
| Clinical data | |
|---|---|
| Trade names | Azaribine |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C14H17N3O9 |
| Molar mass | 371.3 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Azaribine (triacetyl-6-azauridine) is a drug which was developed for the treatment of psoriasis,[1] and also has anti-cancer and antiviral actions.[2][3] It is a prodrug which is metabolised to the nucleoside analogue 6-azauridine in the body. Azaribine was approved for clinical use in treatment of psoriasis,[4] but subsequently withdrawn because of toxicity issues,[5][6] however it continues to be researched as a potential agent for the treatment of emerging viral diseases.[7]
References
- ↑ "New indications for 6-azauridine treatment in man. A review". European Journal of Clinical Pharmacology 4 (2): 77–81. March 1972. doi:10.1007/bf00562501. PMID 4570456.
- ↑ "Clinical and pharmacological studies with 2',3',5'-triacetyl-6-azauridine". Cancer Research 23: 444–53. March 1963. PMID 14023746.
- ↑ "Identification of active antiviral compounds against a New York isolate of West Nile virus". Antiviral Research 55 (1): 107–16. July 2002. doi:10.1016/s0166-3542(02)00013-x. PMID 12076755.
- ↑ "The uses of systemic chemotherapeutic agents in psoriasis". Pharmacology & Therapeutics 14 (1): 1–24. 1981. doi:10.1016/0163-7258(81)90008-5. PMID 7033998.
- ↑ "Azaribine, homocystinemia, and thrombosis". Archives of Dermatology 113 (9): 1301–2. September 1977. doi:10.1001/archderm.1977.01640090149047. PMID 578401.
- ↑ "Evaluation of the characteristics of safety withdrawal of prescription drugs from worldwide pharmaceutical markets-1960 to 1999.". Drug Information Journal 35 (1): 293–317. January 2001. doi:10.1177/009286150103500134.
- ↑ "Identification and characterization of novel compounds with broad spectrum antiviral activity against influenza A and B viruses". Journal of Virology 94 (7). January 2020. doi:10.1128/JVI.02149-19. PMID 31941776.
 |

