Chemistry:Aureothin
| |
| Names | |
|---|---|
| IUPAC name
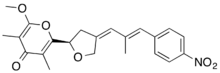
2-methoxy-3,5-dimethyl-6-[(2R,4Z)-4-[(E)-2-methyl-3-(4-nitrophenyl)prop-2-enylidene]oxolan-2-yl]pyran-4-one[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C22H23NO6 | |
| Molar mass | 397.427 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aureothin is a natural product of a cytotoxic shikimate-polyketide antibiotic with the molecular formula C22H23NO6.[2][3][1] Aureothin is produced by the bacterium Streptomyces thioluteus[3][4] that illustrates antitumor, antifungal, and insecticidal activities and the new aureothin derivatives can be antifungal and antiproliferative.[2] In addition, aureothin, a nitro compound from Streptomyces thioluteus, was indicated to have pesticidal activity against the bean weevil[5] by interfering with mitochondrial respiratory complex II.[6]
Biosynthesis
Regarding the biosynthesis of aureothin, the biosynthetic pathway would be begun with chorismic acid. P-nitrobenzoate is derived from p-aminobenzoate by an N-oxygenase, which is encoded by aurF.[7] The aurF is one of the aureothin biosynthetic enzymes and it is referred to as a nonheme diiron oxygenase that is responsible for converting p-aminobenzoate to p-nitrobenzoate.[8] Moreover, the aurF catalyzes a reaction of a complete six-electron oxidation utilizing two equivalents of dioxygen and two exogenous electrons in order to convert p-aminobenzoate to p-nitrobenzoate.[8] Then, three type I Polyketide Synthases (PKSs), which is encoded by aurA, aurB, and aurC, generates the a polyketide chain using p-nitrobenzoate as a starter unit[9] for the biosynthesis of aureothin. At this point, the repetition that one molecule catalyzes two successive cycles of chain extension would occur in the reaction of the type I Polyketide Synthase (PKS).[10] In particular, the two consecutive cycles containing four times of methylmalonyl-CoA and one time of malonyl-CoA occur during the type I Polyketide Synthase (PKS). After O-methylation is activated by a methyltransferase, which is encoded by aurI, the tetrahydrofuran ring formation is produced by a monooxygenase that is encoded by aurH. Therefore, the final product, aureothin, is produced as a result of the monooxygenase encoded by aurH.[11]

References
- ↑ 1.0 1.1 "Aureothin" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/Aureothin#section=Canonical-SMILES.
- ↑ 2.0 2.1 Issues in Chemistry and General Chemical Research (2011 ed.). ScholarlyEditions. 9 January 2012. pp. 442. ISBN 978-1-4649-6334-6.
- ↑ 3.0 3.1 He, Jing; Hertweck, Christian (1 March 2004). "Biosynthetic Origin of the Rare Nitroaryl Moiety of the Polyketide Antibiotic Aureothin: Involvement of an Unprecedented N-Oxygenase". Journal of the American Chemical Society 126 (12): 3694–3695. doi:10.1021/ja039328t. PMID 15038705.
- ↑ Hirata, Yoshimasa; Nakata, Hisao; Yamada, Kiyoyuki; Okuhara, Kunio; Naito, Takayuki (January 1961). "The structure of aureothin, a nitro compound obtained from Streptomyces thioluteus". Tetrahedron 14 (3–4): 252–274. doi:10.1016/S0040-4020(01)92175-1.
- ↑ Oishi, H.; Hosokawa, T.; Okutomi, T.; Suzuki, K. (1969). "Pesticidal Activity of Aureothin". Agricultural and Biological Chemistry 33 (12): 1790–1791. doi:10.1080/00021369.1969.10859541. https://doi.org/10.1080/00021369.1969.10859541.
- ↑ Friedrich, T; Van Heek, P; Leif, H; Ohnishi, T; Forche, E; Kunze, B; Jansen, R; Trowitzsch-Kienast, W et al. (1994). "Two binding sites of inhibitors in NADH: ubiquinone oxidoreductase (complex I). Relationship of one site with the ubiquinone-binding site of bacterial glucose:ubiquinone oxidoreductase". Eur. J. Biochem. 219 (1): 691–698. doi:10.1111/j.1432-1033.1994.tb19985.x. PMID 8307034.
- ↑ He, Jing; Hertweck, Christian (2004). "Biosynthetic Origin of the Rare Nitroaryl Moiety of the Polyketide Antibiotic Aureothin: Involvement of an Unprecedented N-Oxygenase". J. Am. Chem. Soc. 126 (12): 3694–3695. doi:10.1021/ja039328t. PMID 15038705. https://pubs.acs.org/doi/pdf/10.1021/ja039328t.
- ↑ 8.0 8.1 Tsunematsu, Yuta; Hirayama, Yuichiro; Masuya, Takahiro; Watanabe, Kenji (2020). "Oxidative Modification Enzymes in Polyketide Biosynthetic Pathways". Comprehensive Natural Products III (Third Edition) 1: 479–505. doi:10.1016/B978-0-12-409547-2.14637-2. ISBN 9780081026915. https://www.sciencedirect.com/science/article/pii/B9780124095472146372.
- ↑ He, Jing; Hertweck, Christian (2005). "Functional Analysis of the Aureothin Iterative Type I Polyketide Synthase". ChemBioChem 6 (5): 908–912. doi:10.1002/cbic.200400333. PMID 15812854. https://doi.org/10.1002/cbic.200400333.
- ↑ He, Jing; Hertweck, Christian (2004). "Biosynthetic Origin of the Rare Nitroaryl Moiety of the Polyketide Antibiotic Aureothin: Involvement of an Unprecedented N-Oxygenase". J. Am. Chem. Soc. 126 (12): 3694–3695. doi:10.1021/ja039328t. PMID 15038705. https://pubs.acs.org/doi/10.1021/ja039328t.
- ↑ Sugimoto, Yuki; Ishida, Keishi; Traitcheva, Nelly; Busch, Benjamin; Dahse, Hans-Martin; Hertweck, Christian. "Freedom and Constraint in Engineered Noncolinear Polyketide Assembly Lines". ChemBioChem 10 (7): 1225–1232. https://www.cell.com/ccbio/pdfExtended/S1074-5521(15)00004-6.
- ↑ Sakuda, Shohei; Kimura, Makoto (2010). "Toxins of Microorganisms". Comprehensive Natural Products II 4: 411–455. doi:10.1016/B978-008045382-8.00102-7. ISBN 9780080453828. https://www.sciencedirect.com/science/article/pii/B9780080453828001027.
Further reading
- (in en) Natural Product Biosynthesis by Microorganisms and Plants Part C. Academic Press. 31 December 2012. pp. 216. ISBN 978-0-12-404617-7.
- (in en) Strategies and Tactics in Organic Synthesis. Elsevier. 4 October 2016. pp. 120. ISBN 978-0-08-100762-4.
 |

