Chemistry:Arens–van Dorp synthesis
From HandWiki
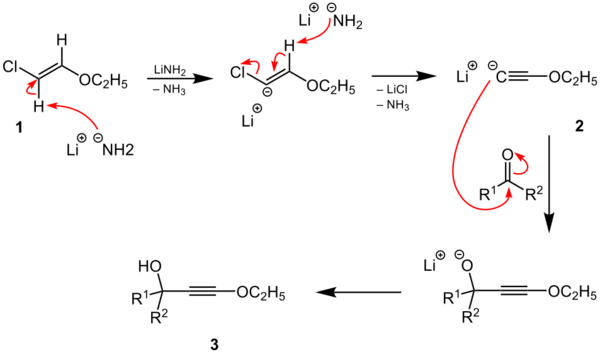
The Arens–van Dorp synthesis is a name reaction in organic chemistry.[1] It describes the addition of lithiated ethoxyacetylenes to ketones to give propargyl alcohols, which can undergo further reaction to form α,β-unsaturated aldehydes,[2] or esters.[3] There is also a variation of this reaction called the Isler modification, where the acetylide anion is generated in situ from β-chlorovinyl ether using lithium amide.[4][5]
References
- ↑ Daniel Zerong Wang (2009). Comprehensive Organic Name Reactions and Reagents. John Wiley & Sons, Inc.. pp. 100–102. ISBN 978-0-471-70450-8.
- ↑ Arens, J. F.; van Dorp, D. A. (1948). "A new method for the synthesis of α,β-unsaturated aldehydes. Preparation of β-methylcinnamic aldehyde, citral and β-ionylidene acetaldehyde". Recueil des Travaux Chimiques des Pays-Bas: 973–979. doi:10.1002/recl.19480671206.
- ↑ Rieder, Curtis J.; Winberg, Karl J.; West, F. G. (2009). "Cyclization of Cross-Conjugated Trienes: The Vinylogous Nazarov Reaction". Journal of the American Chemical Society 131 (22): 7504–7505. doi:10.1021/ja9023226.
- ↑ "Arens–Van Dorp Reaction (Isler Modification)". Comprehensive Organic Name Reactions and Reagents (1st ed.). Hoboken, NJ: Wiley-Interscience. 2009. doi:10.1002/9780470638859.conrr023. ISBN 9780471704508.
- ↑ Van Dorp, D. A.; Arens, J. F. (1947). "Synthesis of Vitamin A Aldehyde-". Nature 160 (4058): 189. doi:10.1038/160189a0. PMID 20256189. Bibcode: 1947Natur.160..189V.
 |