Chemistry:Acrylonitrile
|
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Prop-2-enenitrile | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1093 | ||
| |||
| |||
| Properties | |||
| C3H3N | |||
| Molar mass | 53.064 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Density | 0.81 g/cm3 | ||
| Melting point | −84 °C (−119 °F; 189 K) | ||
| Boiling point | 77 °C (171 °F; 350 K) | ||
| 70 g/L | |||
| log P | 0.19[2] | ||
| Vapor pressure | 83 mmHg[1] | ||
| Hazards | |||
| Main hazards | flammable reactive toxic potential occupational carcinogen[1] | ||
| Safety data sheet | ICSC 0092 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | −1 °C; 30 °F; 272 K | ||
| 471 °C (880 °F; 744 K) | |||
| Explosive limits | 3–17% | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
500 ppm (rat, 4 h) 313 ppm (mouse, 4 h) 425 ppm (rat, 4 h)[3] | ||
LCLo (lowest published)
|
260 ppm (rabbit, 4 h) 575 ppm (guinea pig, 4 h) 636 ppm (rat, 4 h) 452 ppm (human, 1 h)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 2 ppm C 10 ppm [15-minute] [skin][1] | ||
REL (Recommended)
|
Ca TWA 1 ppm C 10 ppm [15-minute] [skin][1] | ||
IDLH (Immediate danger)
|
85 ppm[1] | ||
| Related compounds | |||
Related nitriles
|
acetonitrile propionitrile | ||
Related compounds
|
acrylic acid acrolein | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
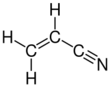
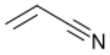
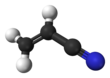
Acrylonitrile is an organic compound with the formula CH
2CHCN and the structure H
2C=CH–C≡N. It is a colorless, volatile liquid. It has a pungent odor of garlic or onions.[4] Its molecular structure consists of a vinyl group (–CH=CH
2) linked to a nitrile (–C≡N). It is an important monomer for the manufacture of useful plastics such as polyacrylonitrile. It is reactive and toxic at low doses.[5]
Acrylonitrile is one of the components of ABS plastic (Acrylonitrile butadiene styrene).[6]
Structure and basic properties
Acrylonitrile is an organic compound with the formula CH
2CHCN and the structure H
2C=CH–C≡N. It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions.[4]
Its molecular structure consists of a vinyl group (–CH=CH
2) linked to a nitrile (–C≡N). It is an important monomer for the manufacture of useful plastics such as polyacrylonitrile. It is reactive and toxic at low doses.[5]
Production
Acrylonitrile was first synthesized by the French chemist Charles Moureu in 1893.[7] Acrylonitrile is produced by catalytic ammoxidation of propylene, also known as the SOHIO process. In 2002, world production capacity was estimated at 5 million tonnes per year,[5][8] rising to about 6 million tonnes by 2017.[9] Acetonitrile and hydrogen cyanide are significant byproducts that are recovered for sale.[5] In fact, the 2008–2009 acetonitrile shortage was caused by a decrease in demand for acrylonitrile.[10]
- 2 CH
3–CH=CH
2 + 2 NH
3 + 3 O
2 → 2 CH
2=CH–C≡N + 6 H
2O
In the SOHIO process, propylene, ammonia, and air (oxidizer) are passed through a fluidized bed reactor containing the catalyst at 400–510 °C and 50–200 kPag. The reactants pass through the reactor only once, before being quenched in aqueous sulfuric acid. Excess propylene, carbon monoxide, carbon dioxide, and dinitrogen that do not dissolve are vented directly to the atmosphere, or are incinerated. The aqueous solution consists of acrylonitrile, acetonitrile, hydrocyanic acid, and ammonium sulfate (from excess ammonia). A recovery column removes bulk water, and acrylonitrile and acetonitrile are separated by distillation. One of the first useful catalysts was bismuth phosphomolybdate (Bi
9PMo
12O
52) supported on silica.[11] Further improvements have since been made.[5]
Alternative routes
Various green chemistry routes to acrylonitrile are being explored from renewable feedstocks, such as lignocellulosic biomass, glycerol (from biodiesel production), or glutamic acid (which can itself be produced from renewable feedstocks). The lignocellulosic route involves fermentation of the biomass to propionic acid and 3-hydroxypropionic acid, which are then converted to acrylonitrile by dehydration and ammoxidation.[12][9] The glycerol route begins with its dehydration to acrolein, which undergoes ammoxidation to give acrylonitrile.[13] The glutamic acid route employs oxidative decarboxylation to 3-cyanopropanoic acid, followed by a decarbonylation-elimination to acrylonitrile.[14] Of these, the glycerol route is broadly considered to be the most viable, although none of these green methods are commercially competitive.[12][13]
Uses
Acrylonitrile is used principally as a monomer to prepare polyacrylonitrile, a homopolymer, or several important copolymers, such as styrene-acrylonitrile (SAN), acrylonitrile butadiene styrene (ABS), acrylonitrile styrene acrylate (ASA), and other synthetic rubbers such as acrylonitrile butadiene (NBR). Hydrodimerization of acrylonitrile[15][16] affords adiponitrile, used in the synthesis of certain nylons:
- 2 CH
2=CHCN + 2 e−
+ 2 H+
→ NCCH
2–CH
2–CH
2–CH
2CN
Acrylonitrile is also a precursor in the manufacture of acrylamide and acrylic acid.[5]
Synthesis of chemicals
Hydrogenation of acrylonitrile is one route to propionitrile. Hydrolysis with sulfuric acid gives acrylamide sulfate, CH=CHC(O)NH
2 · H
2SO
4. This salt can be converted to acrylamide with treatment with base or to methyl acrylate by treatment with methanol.[5]
The reaction of acrylonitrile with protic nucleophiles is a common route to a variety of specialty chemicals. The process is called cyanoethylation:
- YH + H
2C=CHCN → Y–CH
2–CH
2CN
Typical protic nucleophiles are alcohols, thiols, and especially amines.[17]
Acrylonitrile and derivatives, such as 2-chloroacrylonitrile, are dienophiles in Diels–Alder reactions.
Health effects
Acrylonitrile is moderately toxic with LD50 = 81 mg/kg (rats). It undergoes explosive polymerization. The burning material releases fumes of hydrogen cyanide and oxides of nitrogen. It is classified as a Class 2B carcinogen (possibly carcinogenic) by the International Agency for Research on Cancer (IARC),[18] and workers exposed to high levels of airborne acrylonitrile are diagnosed more frequently with lung cancer than the rest of the population.[19] Acrylonitrile is one of seven toxicants in cigarette smoke that are most associated with respiratory tract carcinogenesis.[20] The mechanism of action of acrylonitrile appears to involve oxidative stress and oxidative DNA damage.[21] Acrylonitrile increases cancer in high dose tests in male and female rats and mice[22] and induces apoptosis in human umbilical cord mesenchymal stem cells.[23]
It evaporates quickly at room temperature (20 °C) to reach dangerous concentrations; skin irritation, respiratory irritation, and eye irritation are the immediate effects of this exposure.[24] Pathways of exposure for humans include emissions, auto exhaust, and cigarette smoke that can expose the human subject directly if they inhale or smoke. Routes of exposure include inhalation, oral, and to a certain extent dermal uptake (tested with volunteer humans and in rat studies).[25] Repeated exposure causes skin sensitization and may cause central nervous system and liver damage.[24]
There are two main excretion processes of acrylonitrile. The primary method is excretion in urine when acrylonitrile is metabolized by being directly conjugated to glutathione. The other method is when acrylonitrile is enzymatically converted into 2-cyanoethylene oxide which will produce cyanide end products that ultimately form thiocyanate, which is excreted via urine.[25] Exposure can thus be detected via blood draws and urine sampling.[18]
Incidents
A large amount of acrylonitrile (approximately 6500 tons) leaked from an industrial polymer plant owned by Aksa Akrilik after the violent 17 August earthquake in Turkey. Over 5000 people were affected and the exposed animals had died.[26] The leak was only noticed by the company 8 hours after the incident. Healthcare workers did not know about the health effects of acrylonitrile and tried to treat the victims with painkillers and IV fluids.[27] One lawyer, Ayşe Akdemir, sued the company with 44 families as the plaintiffs.[27] Aksa Akrilik was sued by 200 residents who were affected by acrylonitrile.[28] An increase in cancer cases in the area was confirmed by the Turkish Medical Association,[28] as the cancer rate in the affected area has increased by 80%, from 1999 to April 2002.[27] In 2003, the owner of Aksa Akrilik has died from lung cancer related to acrylonitrile exposure.[27] As of 2001, this is the largest acrylonitrile leak known.[26]
Occurrence
Acrylonitrile is not naturally formed on Earth. It has been detected at the sub-ppm level at industrial sites. It persists in the air for up to a week. It decomposes by reacting with oxygen and hydroxyl radical to form formyl cyanide and formaldehyde.[29] Acrylonitrile is harmful to aquatic life.[24] Acrylonitrile has been detected in the atmosphere of Titan, a moon of Saturn.[30][31][32] Computer simulations suggest that on Titan conditions exist such that the compound could form structures similar to cell membranes and vesicles on Earth, called azotosomes.[30][31]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 NIOSH Pocket Guide to Chemical Hazards. "#0014". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0014.html.
- ↑ "Acrylonitrile_msds". https://www.chemsrc.com/en/cas/107-13-1_1186893.html.
- ↑ 3.0 3.1 "Acrylonitrile". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/107131.html.
- ↑ 4.0 4.1 "Medical Management Guidelines for Acrylonitrile". Agency for Toxic Substances & Disease Registry. https://www.atsdr.cdc.gov/mmg/mmg.asp?id=443&tid=78.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Brazdil, James F.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_177.pub3.
- ↑ Campo, E. Alfredo (2008-01-01), Campo, E. Alfredo, ed., "1 - Polymeric Materials and Properties", Selection of Polymeric Materials, Plastics Design Library (Norwich, NY: William Andrew Publishing): pp. 1–39, doi:10.1016/b978-081551551-7.50003-6, ISBN 978-0-8155-1551-7, https://www.sciencedirect.com/science/article/pii/B9780815515517500036, retrieved 2023-11-20
- ↑
- Moureu, C. (1893). "Contribution à l'étude de l'acide acrylique et de ses dérivés". Annales de chimie et de physique. 7th 2: 145–212. http://babel.hathitrust.org/cgi/pt?id=uc1.31822017842394;view=1up;seq=149. See especially pp. 187–189 ("Nitrile acrylique ou cyanure de vinyle (Propène-nitrile)").
- Moureu, C. (1893). "Nitrile acrylique, cyanure de vinyle (propène-nitrile)". Bulletin de la Société Chimique de France. 3rd 9: 424–427. http://gallica.bnf.fr/ark:/12148/bpt6k282008w/f436.image.r=BulletindelaSocieteChimiquedeParis.langFR.
- ↑ "The Sohio Acrylonitrile Process". American Chemical Society National Historic Chemical Landmarks. http://portal.acs.org/portal/PublicWebSite/education/whatischemistry/landmarks/acrylonitrile/index.htm.
- ↑ 9.0 9.1 Davey, Stephen G. (January 2018). "Sustainability: Sweet new route to acrylonitrile". Nature Reviews Chemistry 2 (1): 0110. doi:10.1038/s41570-017-0110.
- ↑ Tullo, A. (2008). "A Solvent Dries Up". Chemical & Engineering News 86 (47): 27. doi:10.1021/cen-v086n047.p027.
- ↑ Grasselli, Robert K. (2014). "Site isolation and phase cooperation: Two important concepts in selective oxidation catalysis: A retrospective" (in en). Catalysis Today 238: 10–27. doi:10.1016/j.cattod.2014.05.036.
- ↑ 12.0 12.1 Grasselli, Robert K.; Trifirò, Ferruccio (2016). "Acrylonitrile from Biomass: Still Far from Being a Sustainable Process". Topics in Catalysis 59 (17–18): 1651–1658. doi:10.1007/s11244-016-0679-7. ISSN 1022-5528.
- ↑ 13.0 13.1 Guerrero-Pérez, M. Olga; Bañares, Miguel A. (2015). "Metrics of acrylonitrile: From biomass vs. petrochemical route". Catalysis Today 239: 25–30. doi:10.1016/j.cattod.2013.12.046. ISSN 0920-5861.
- ↑ Le Nôtre, Jérôme; Scott, Elinor L.; Franssen, Maurice C. R.; Sanders, Johan P. M. (2011). "Biobased synthesis of acrylonitrile from glutamic acid". Green Chemistry 13 (4): 807. doi:10.1039/c0gc00805b. ISSN 1463-9262.
- ↑ Ellis, Paul G (1972). A radiation-chemical study of the hydrodimerisation of acrylonitrile. UK: Leeds University, Ph D thesis.
- ↑ Buxton, George V.; Ellis, Paul G.; McKillop, Thomas F.W. (1979). "Pulse radiolysis study of acrylonitrile in aqueous solution". J. Chem. Soc., Faraday Trans. 1 75: 1050. doi:10.1039/f19797501050.
- ↑ Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001.
- ↑ 18.0 18.1 "Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide". IARC Monographs, Volume 71 (1999)
- ↑ Acrylonitrile Fact Sheet (CAS No. 107-13-1). epa.gov
- ↑ Cunningham FH, Fiebelkorn S, Johnson M, Meredith C. A novel application of the Margin of Exposure approach: segregation of tobacco smoke toxicants. Food Chem Toxicol. 2011 Nov;49(11):2921-33. doi: 10.1016/j.fct.2011.07.019. Epub 2011 Jul 23. PMID 21802474
- ↑ Pu X, Kamendulis LM, Klaunig JE. Acrylonitrile-induced oxidative stress and oxidative DNA damage in male Sprague-Dawley rats. Toxicol Sci. 2009;111(1):64-71. doi:10.1093/toxsci/kfp133
- ↑ "Acrylonitrile: Carcinogenic Potency Database".
- ↑ Sun, X. (January 2014). "Cytotoxic effects of acrylonitrile on human umbilical cord mesenchymal stem cells in vitro". Molecular Medicine Reports 9 (1): 97–102. doi:10.3892/mmr.2013.1802. PMID 24248151. http://www.spandidos-publications.com/mmr/9/1/97.
- ↑ 24.0 24.1 24.2 "CDC – Acrylonitrile – International Chemical Safety Cards". NIOSH. https://www.cdc.gov/niosh/ipcsneng/neng0092.html.
- ↑ 25.0 25.1 Acrylonitrile Fact Sheet: Support Document (CAS No. 107-13-1). epa.gov
- ↑ 26.0 26.1 Nadi Bakırcı (2001). "ENDÜSTRİYEL BİR ÇEVRE FELAKETİ: AKRİLONİTRİL". Turkish Medical Association. https://www.ttb.org.tr/msg/dergi/ocak01/9.htm.
- ↑ 27.0 27.1 27.2 27.3 Fatma Dalokay (30 November 2020). "17 Ağustos 1999 Depremi: Akrilonitril Zehirlenmesi". https://tabella.org/2020/11/30/17-agustos-1999-depremi-akrilonitril-zehirlenmesi/.
- ↑ 28.0 28.1 "İSO'nun şaşırtan çevre ödülü Aksa'nın". 26 June 2005. https://mbigpara.hurriyet.com.tr/amp/haberler/iso-nun-sasirtan-cevre-odulu-aksa-nin/525904/.
- ↑ Grosjean, Daniel (December 1990). "Atmospheric Chemistry of Toxic Contaminants. 3. Unsaturated Aliphatics: Acrolein, Acrylonitrile, Maleic Anhydride". Journal of the Air & Waste Management Association 40 (12): 1664–1669. doi:10.1080/10473289.1990.10466814. Bibcode: 1990JAWMA..40.1664G.
- ↑ 30.0 30.1 Wall, Mike (28 July 2017). "Saturn Moon Titan Has Molecules That Could Help Make Cell Membranes". Space.com. https://www.space.com/37653-saturn-moon-titan-cell-membrane-molecules.html.
- ↑ 31.0 31.1 Palmer, Maureen Y. (28 July 2017). "ALMA detection and astrobiological potential of vinyl cyanide on Titan". Science Advances 3 (7): e1700022. doi:10.1126/sciadv.1700022. PMID 28782019. Bibcode: 2017SciA....3E0022P.
- ↑ Kaplan, Sarah (8 August 2017). "This weird moon of Saturn has some essential ingredients for life". The Washington Post. https://www.washingtonpost.com/news/speaking-of-science/wp/2017/08/08/this-weird-moon-of-saturn-has-some-essential-ingredients-for-life/.
External links
- National Pollutant Inventory – Acrylonitrile
- Comparing Possible Cancer Hazards from Human Exposures to Rodent Carcinogens
- Acrylonitrile – Integrated Risk Information System, U.S. Environmental Protection Agency
- CDC – NIOSH Pocket Guide to Chemical Hazards – Acrylonitrile
- OSHA Table Z-1 for Air Contaminants
 |