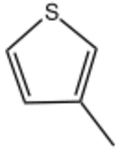
Chemistry:3-Methylthiophene
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Methylthiophene | |
| Other names
3-Thiotolene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H6S | |
| Molar mass | 98.16 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.016 g/cm3 |
| Melting point | −69 °C (−92 °F; 204 K) |
| Boiling point | 114 °C (237 °F; 387 K) |
| 0.4 g/l | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H225, H302, H312, H315, H319, H332, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+312, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P312, P321, P322, P330, P332+313, P337+313, P362, P363 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
3-Methylthiophene is an organosulfur compound with the formula CH3C4H3S. It is a colorless, flammable liquid. It can be produced by sulfidation of 2-methylsuccinate.[1] Like its isomer 2-methylthiophene, its commercial synthesis involvess vapor-phase dehydrogenation of suitable precursors. 3-Methylthiophene is a precursor to the drug thenyldiamine and the pesticide morantel.[2]
References
- ↑ R. F. Feldkamp; B. F. Tullar (1954). "3-Methylthiophene". Org. Synth. 34: 73. doi:10.15227/orgsyn.034.0073.
- ↑ Swanston, Jonathan (2006). "Thiophene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_793.pub2. ISBN 3527306730..
 |

