Chemistry:3,3-Dimethylpentane
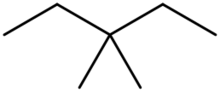 Skeletal structure of 3,3-dimethylpentane
| |
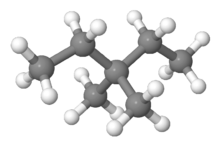 Ball-and-Stick model of 3,3-dimethylpentane
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,3-Dimethylpentane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H16 | |
| Molar mass | 100.205 g·mol−1 |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H225, H304, H315, H336, H410 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P273, P280, P301+310, P302+352, P303+361+353, P304+340, P312, P321, P331, P332+313, P362, P370+378, P391, P403+233, P403+235, P405 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3,3-Dimethylpentane is one of the isomers of heptane. 3,3-Dimethylpentane has a boiling point of 86.0 °C and melting point of −134.9 °C. Its density is 0.6934. The refractive index is 1.39092 at 20 °C.[1]
Preparation
A method to produce 3,3-dimethylpentane is to react tert-amyl chloride (CH3CH2(CH3)C2Cl) with propionaldehyde producing 3,3-dimethylpentan-2-ol. This is then dehydrated to produce 3,3-dimethylpent-2-ene, which when hydrogenated produces some 3,3-dimethylpentane, but also 2,3-dimethylpentane.[2]
Properties
In 1929 Graham Edgar and George Calingaert made 3,3-dimethylpentane and measured its physical characteristics for the first time. The measurements were at 20 °C, not the standard conditions used in later times.[3]
For 3,3-dimethylpentane they measured a density of 0.6934 at 20 °C with a rate of change Δd/ΔT of 0.000848. The dielectric constant is 1.940. The refractive index at 20° is 1.39114. The adiabatic compressibility is 0.00011455 and isothermal compressibility is 0.00014513 atmospheres. The velocity of sound is 1.1295 km/s. Coefficient of thermal expansion is 0.002467/°. Surface tension is 19.63 dynes/cm. Viscosity is 0.00454. The heat of combustion is 11470 cal/g which is very similar to other heptanes.[3]
References
- ↑ Ferris, S. W. (2018) (in en). Handbook of Hydrocarbons. Elsevier. p. 24. ISBN 978-1-4832-7285-6. https://books.google.com/books?id=0yoSBQAAQBAJ&pg=PA24.
- ↑ Edgar, Graham; Calingaert, George; Marker, R. E. (May 1929). "The Preparation And Properties Of The Isomeric Heptanes. Part I. Preparation". Journal of the American Chemical Society 51 (5): 1483–1491. doi:10.1021/ja01380a027.
- ↑ 3.0 3.1 Edgar, Graham; Calingaert, George (May 1929). "The Preparation And Properties Of The Isomeric Heptanes. Part II. Physical Properties". Journal of the American Chemical Society 51 (5): 1540–1550. doi:10.1021/ja01380a035.
 |

