Chemistry:1-Naphthoic acid
From HandWiki
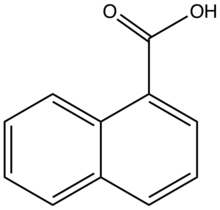
| |
| Names | |
|---|---|
| Preferred IUPAC name
Naphthalene-1-carboxylic acid | |
| Other names
1-Naphthylenecarboxylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1908896 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 28651 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H8O2 | |
| Molar mass | 172.183 g·mol−1 |
| Appearance | white solid |
| Melting point | 161 °C (322 °F; 434 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1-Naphthoic acid is an organic compound of the formula C10H7CO2H. It is one of two isomeric monocarboxylic acids of naphthalene, the other one being 2-naphthoic acid. 1-Naphthoic acid is a frequent substrate for C-H activation reactions.[1] In general the hydroxynaphthoic acids are far more useful than the parent. It can be prepared by carboxylation of the Grignard reagent generated from 1-Bromonaphthalene.[2]
References
- ↑ Mochida, Satoshi; Hirano, Koji; Satoh, Tetsuya; Miura, Masahiro (2011). "Rhodium-Catalyzed Regioselective Olefination Directed by a Carboxylic Group". The Journal of Organic Chemistry 76 (9): 3024–3033. doi:10.1021/jo200509m. PMID 21438629.
- ↑ "α-Naphthoic Acid". Organic Syntheses 11: 80. 1931. doi:10.15227/orgsyn.011.0080.
 |

